Received: Tue 02, Apr 2024
Accepted: Wed 22, May 2024
Abstract
Background: Placenta accreta spectrum (PAS) is a life-threatening obstetric condition worldwide, accompanied by multiple complications, and its severity is difficult and objective to diagnose. We aimed to develop an intraoperative scoring system to diagnose severe PAS and predict severe complications.
Methods: Patients with placenta previa were retrospectively enrolled to analyze the risk factors closely related to massive postpartum hemorrhage (PPH) and construct a risk model of the intraoperative scoring system by logistic regression analysis, Delphi method and validated by Hosmer-Lemeshow test, which was also prospectively verified among PAS patients by the receiver operating characteristic (ROC) curve. Kappa test was used to evaluate the agreement level between scoring system and external data.
Results: After the binary logistic regression analysis of 493 retrospective data points, there were 3 independent high-risk factors related to massive PPH including anterior placental location, complete PP, and placental adhesion (also divided into the invading depth of the muscle layer, the diameter of the invading area, invading part and neovascularization density), and 6 items in total after three Delphi rounds. The model was used to predict among 112 cases of PAS when the score was 6.5, high-risk PAS could be diagnosed during surgery, yielding an area under the ROC curve of 0.939 (95% CI, 0.891-0.987, P<0.001), with a sensitivity of 94.2% and specificity of 85%. If the score was ≥ 12.5, a hysterectomy would probably be applied, with a ROC curve of 0.945 (95% CI, 0.885-1.000, P<0.001), a sensitivity of 90.9%, and a specificity of 95%. For two other complications of disseminated intravascular coagulation (DIC) and massive blood transfusion, their cutoff value was 8.5. External validation showed a relative high Kappa value of 0.744 (95% CI, P<0.001, 0.595-0.982) for high-risk PAS.
Conclusion: We first proposed an intraoperative scoring system for diagnosing high-risk PAS and predicting its serious complications.
Keywords
Scoring system, placenta accreta spectrum, intraoperative, preoperative, diagnose, complications
1. Introduction
The placenta accreta spectrum (PAS), including placenta accreta, increta, and percreta, is a life-threatening obstetric condition that can cause massive postpartum hemorrhage (PPH) [1]. The incidence of PAS disorders has increased from 1 per 4000 deliveries in the 1970s to 1 per 272 deliveries in the last decade [2]. PAS cases can have many short-term complications, including PPH, disseminated intravascular coagulation (DIC), damage to adjacent organs, cesarean hysterectomy, and long-term psychological sequelae due to loss of femininity and fertility [3, 4].
Prenatal prediction of PAS can minimize these complications and enable sufficient surgical preparation by obstetricians, such as the assembly of a multidisciplinary team, determination of the anesthesia mode, preservation of blood products, and preparation for interventional radiology for uterine artery embolization (UAE) or balloon occlusion [5]. Previous studies have demonstrated that some clinical risk factors and sonographic findings are important to evaluate the severity of PAS. These clinical risk factors include maternal age, fetal presentation, and number of C-sections, among others [6, 7]. Sonographic findings include multiple lacunae, loss of the hypoechoic placenta-uterine demarcation line, placental position and interruption of the bladder-uterine interface, among others [8-10].
Although dedicated ultrasound and magnetic resonance imaging (MRI) have improved the antenatal diagnosis, between one-half and two-thirds of cases remain undiagnosed, resulting in poorer maternal outcomes [11-13]. The severity of PAS could be confirmed only postoperatively by the surgeons’ clinical impression and pathological outcome. However, not all PAS cases have pathological results [14].The final diagnosis of PAS severity is difficult and subjective. We attempted to develop an intraoperative scoring system that is more objective in diagnosing high-risk PAS. The scoring items were chosen based on retrospective findings from cases with placenta previa, and the items were closely related to massive PPH. We also evaluate the scoring system by internal and external validation.
2. Materials and Methods
2.1. General Information Collection of the Retrospective Study
The clinical data from pregnant women with placenta previa (PP) in our hospital from October 2012 to March 2019 were collected. The retrospective registration number was ChiCTR2000035286. Informed consent was waived due to the retrospective nature of the study. The clinical records of the mothers and babies were reviewed, and the risk factors for massive PPH were analyzed by binary logistical regression. Massive (or severe) PPH was defined as estimated blood loss ≥ 2000 mL [15], and massive blood transfusion was defined as blood transfusion ≥ a 10-µ package of red blood cells in 24 hours [16]. PP was diagnosed by experienced technicians based on transvaginal ultrasound findings showing that the placenta covered the internal cervix os after 20 gestational weeks. Prenatal diagnosis was confirmed within 1 week before delivery. A diagnosis of “complete PP” was made when the internal cervical os was completely covered by the placenta, while “incomplete PP” was diagnosed when the internal cervical os was partially covered, with a low-lying placenta (< 2 cm).
Clinical data were divided into three parts: i) Obstetrical risk factors (maternal age, gravidity, parity, number of intrauterine procedures, number of fetuses in the current pregnancy, gestational age at termination, number of previous C-sections, proportion of multipara, and type of PP); ii) Maternal outcomes (delivery mode, PPH, usage of blood products, hysterectomy,); and iii) Neonatal outcomes (neonatal sex, birth weight, Apgar score, fetal presentation, neonatal asphyxia, and neonatal intensive care unit (NICU) admission). The number of intrauterine procedures refers to the total number of intrauterine operations, such as artificial abortion, hysteroscopy and other related procedures ever undergone on the patient.
2.2. General Information Collection of the Prospective Study
To study the preoperative and intraoperative scoring systems, patients with PP were prospectively enrolled from April 2019 to March 2020. The work has been reported in accordance with the STROCSS criteria [17]. PAS was ultimately diagnosed by two experienced technicians based on transvaginal ultrasound after 24 gestational weeks. The clinical data of the 112 patients were also collected and divided into three parts: i) Obstetrical risk factors (maternal age, gravidity, parity, number of intrauterine procedures, number of fetuses in the current pregnancy, gestational age at termination, number of previous C-sections, proportion of multipara, and type of PP); ii) Maternal outcomes (delivery mode, PPH, usage of blood products, hysterectomy, and ICU admission); and iii) Neonatal outcomes (neonatal sex, birth weight, Apgar score, fetal presentation, neonatal asphyxia, and NICU admission). They were given scores per preoperative and intraoperative scoring systems. All procedures performed in studies involving human participants were registered in the Chinese Clinical Trial Registry Center. The prospective registration number was ChiCTR200003526. Informed consent was given in the prospective study.
2.3. Instructions for Delphi Panel of Experts
To develop the scoring system, 8 obstetrical experts from Jiangsu Province employed the rigorous Delphi method, a structured communication technique aimed at achieving consensus among a panel of experts. Initially, the experts individually formulated and refined a set of criteria deemed essential for the scoring system based on the literature searched in PubMed, Google Scholar, Web of Science, EMBASE, and Cochrane library. These criteria encompassed various aspects pertinent to the intended application. Through a series of iterative rounds, the experts anonymously exchanged feedback, critiqued each other’s suggestions, and revised the criteria accordingly. This iterative process continued until a consensus was reached on the final set of criteria. The Delphi method facilitated a systematic and collaborative approach, harnessing the collective expertise of the panel to ensure the robustness and validity of the scoring system.
2.4. High-Risk PAS Diagnostic Criteria
Total blood loss (TBL) was used to help evaluate PAS severity. PAS was diagnosed postoperatively, which was considered a standard criterion: high-risk PAS was characterized by TBL ≥ 2000 mL, application of UAE, balloon occlusion, hysterectomy, or DIC), with TBL = blood loss 1 + blood loss 2. Blood loss 1 was calculated using the following formula: blood loss (mL) = (blood clothing or sanitary napkins - dry clothes or sanitary napkins) / 1.05. Blood loss 2 was calculated from the volume of blood suctioned during C-section excluding amniotic fluid.
2.5. General Information Collection of the External Data
To demonstrate this score model, we collaborate with another three hospitals, include Huai'an Maternity and Child Healthcare Hospital, Changzhou No 2 People’s Hospital and Taixing People's Hospital, from August 2022 to August 2023. The relative clinical data were collected in accordance with the inclusion criteria of PAS as above mentioned.
2.6. Statistical Methods
Data were analyzed using the statistical software SPSS 24.0 (SPSS Inc., Chicago, IL, USA). The normality of continuous variables was analyzed by a Shapiro-Wilk test. The standard normally distributed data are described as the means ± standard deviation (SD) and were compared by student’s t test. Nonnormally distributed variables are expressed as the medians (interquartile range) and were compared by a Mann-Whitney U test. Categorical variables were described as concrete cases (percentages) and compared by a chi-square test or Fisher’s exact test. Logistic regression analysis was used to determine which clinical parameters were significantly associated with massive PPH and validated by Hosmer-Lemeshow test. ROC curves were used to predict the model among prospective 112 cases of high-risk PAS, hysterectomy, DIC and massive blood transfusion. The level of agreement of the scoring system with external data was assessed using the kappa test. P <0.05 was considered statistically significant.
3. Results
3.1. Obstetric Risk Factors for Massive PPH in Placenta Previa Patients
In total, 493 patients were recruited in this retrospective study. Table 1 shows the comparison of clinical features between the massive PPH and control groups. There were no significant differences in terms of the number of intrauterine procedures, gestational age at termination, emergency operation, birth weight, Apgar score, number of fetuses, male newborns, or asphyxia (P>0.05). Maternal age, gravidity, and C-sections history were significantly higher in the massive PPH group than in the control group. The incidence of complete PP (P<0.001), anterior placenta, placenta adhesion, hysterectomy and NICU admission were significantly higher in the massive PPH group than in the control group.
Table
1: Clinical Features
of control and massive PPH in placenta previa groups.
|
Clinical
Features |
Control (n=321,%) |
Massive
PPH (n=172,%) |
P
value |
|
Maternal
Age (years) |
30 (27~35) |
32 (28~36) |
0.041 |
|
Gravidity
(times) |
3 (2~4) |
3 (2~4) |
0.001 |
|
No.
of intrauterine procedures (times) |
1 (0~2) |
1 (1~2) |
0.103 |
|
Times
of c-section (times) |
|||
|
0 |
175 (54.5) |
50 (29.1) |
<0.001 |
|
1 |
88 (27.4) |
81 (47.1) |
<0.001 |
|
2 |
58 (18.1) |
41 (23.8) |
>0.05 |
|
GA termination
(weeks) |
34.4 (36.3~37.7) |
36.0 (34.3~37.4) |
0.263 |
|
Birth
weight (g) |
2770.81±664.21 |
2711.92±608.13 |
0.335 |
|
APGAR
1min |
|||
|
8~10 |
285 (88.8) |
146 (84.9) |
0.432 |
|
4~7 |
29 (9.0) |
20 (11.6) |
|
|
0~3 |
7 (2.2) |
6 (3.5) |
|
|
APGAR
5min |
|||
|
8~10 |
310 (96.6) |
162 (94.2) |
0.454 |
|
4~7 |
9 (2.8) |
8 (4.7) |
|
|
0~3 |
2 (0.6) |
2 (1.2) |
|
|
Emergency
operation (n) |
77 (24) |
53 (30.8) |
0.101 |
|
Antepartum
bleeding (n) |
119 (37.1) |
61 (35.5) |
0.724 |
|
Complete
placenta previa (n) |
184 (57.3) |
149 (86.6) |
<0.001 |
|
Anterior
placenta (n) |
117 (36.4) |
146 (85.4) |
<0.001 |
|
Placenta
adhesion (n) |
42 (13.1) |
113 (65.7) |
<0.001 |
|
Hysterectomy
(n) |
0 |
19 (11.0) |
<0.001 |
|
Twins
(n) |
7 (2.2) |
4 (2.3) |
1.000 |
|
Male
newborn (n) |
148 (46.1) |
88 (51.2) |
0.284 |
|
Asphyxia
(n) |
36 (11.2) |
23 (13.4) |
0.482 |
|
NICU
(n) |
72 (22.4) |
57 (33.1) |
0.010 |
All clinical characteristics were divided into three models and analyzed by binary logistic regression. The first model included maternal age, gravidity, number of intrauterine procedures, number of C-sections, gestational age, operation type, and antepartum hemorrhage. The second model included newborn sex, birth weight, fetal presentation and number of fetuses. The third model included placental location, PP type and placental adhesion. In the first model, the number of gravidities and number of C-sections significantly increased the risk of massive PPH. The ORs for massive PPH were 2.270 (95% CI, 1.186-4.347, P=0.013) and 2.578 (95% CI, 1.318-5.040, P=0.006) when the numbers of pregnancies were 4 and ≥5, respectively, compared to 1 pregnancy. Regarding the number of C-sections, the OR (≥2 C-sections) was 1.647 (95% CI, 1.286-2.109, P <0.001) compared with no C-section. When the second model was added, the number of C-sections and fetal presentation were significant.
The OR (≥2 C-sections) was 1.596 (95% CI, 1.235-2.063, P <0.001) compared with only one C-section. The OR of breech presentation was 4.202 (95% CI, 2.534-6.968, P <0.001) compared with cephalic presentation. Furthermore, the third model was added, and the final significant factors, including placental location, PP type and placental adhesion, were entered. The anterior placental location had an OR of 8.318 (95% CI, 4.718-14.665, P<0.001) compared to the nonanterior placenta. Complete PP had an OR of 2.460 (95% CI, 1.303-4.643, P=0.005) compared with incomplete PP. The OR of PP with placental adhesion was 10.013 (95% CI, 5.530-18.129, P<0.001) compared with PP without adhesion (Table 2).
Table
2: Analysis of
obstetric risk factors for Massive PPH in placenta previa group by binary
logistic regression analysis.
|
Models |
Control (n=321, %) |
Massive
PPH (n=172, %) |
OR(95%CI)
|
P
value
|
|
|
Model
1 |
|||||
|
Gravidity |
|||||
|
4 |
53 (16.5) |
39 (22.7) |
2.270 (1.186-4.347) |
0.013 |
|
|
>=5 |
44 (13.7) |
35 (20.3) |
2.578(1.318-5.040) |
0.006 |
|
|
Times
of C-section |
|||||
|
>=2 |
58 (18.1) |
41 (23.8) |
1.647 (1.286-2.109) |
<0.001 |
|
|
Model
2 |
|||||
|
Times
of C-section |
|
|
|
|
|
|
>=2 |
58 (18.1) |
41 (23.8) |
1.596 (
1.235-2.063) |
<0.001 |
|
|
Fetal
Presentation |
|
|
|
|
|
|
Breech |
34 (10.6) |
61 (35.5) |
4.202 (
2.534-6.968) |
<0.001 |
|
|
Model
3 |
|||||
|
Placental
Location |
|||||
|
Anterior |
117 (36.4) |
146 (85.4) |
8.318 (
4.718-14.665) |
<0.001 |
|
|
Placenta
Previa Type |
|||||
|
Complete
PP |
184 (57.3) |
149 (86.6) |
2.460 (1.303-4.643) |
0.005 |
|
|
Placenta
Adhesion |
|||||
|
Yes |
42 (13.1) |
113 (65.7) |
10.013
( 5.530-18.129) |
<0.001 |
|
P <0.05 was considered
statistically significant.
3.2. Development of an Intraoperative Scoring System
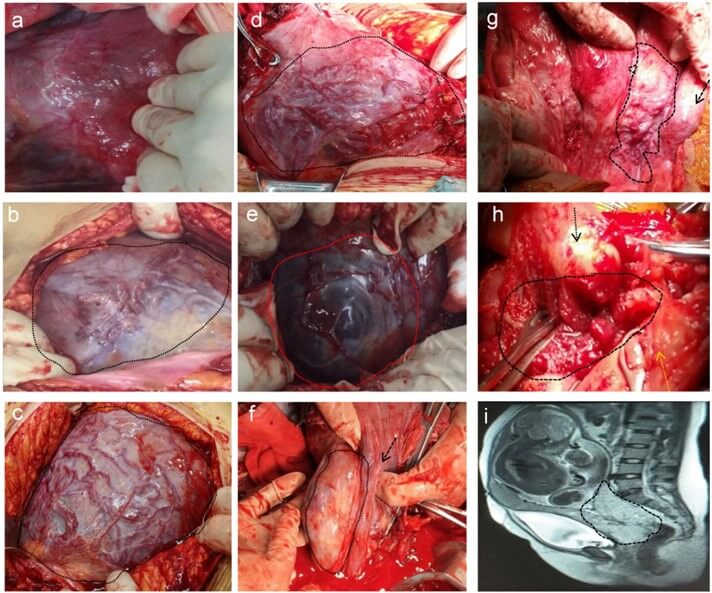
We proposed an intraoperative scoring system (Table 3) by Delphi method. Each criterion was assigned 0, 1 or a maximum of 3 points, and the sum of the points obtained from each criterion yielded the final score. Variables and scores in the scoring system were as follows: placental location (0 points for nonanterior wall, 1 point for anterior wall); relationship between the placenta and cervix os (0 points for a low-lying placenta or partially covered cervix, 1 point for fully covered cervix); invading depth of the muscle layer (0 points for no invasion, 1 point for depth <1/2, 2 points for depth ≥ 1/2, 3 points for full thickness); diameter of the invading area (0 points for 0 cm, 1 point for <3 cm, 2 points for 3-5 cm, 3 points for ≥ 5 cm); neovascularization density in the serosal surface of the lower segment of the uterus (0 points without neovascularization, 1 point for <30%, 2 points for 30-50%, 3 points for ≥ 50%); and location of invasion (0 points for invasion confined to the uterus, 3 points for invasion in the cervical canal and outside of the uterus, including the bladder, rectum, and vaginal pelvic region). Figure 1a-1d show some examples of neovascularization density in the serosal surface of the lower segment of the uterus. Figure 1e-1j show some examples of different invading parts, the uterus (Figure 1e), extrauterine region (Figure 1f & 1g) and cervix (Figure 1h), (Figure 1i) is the MRI image of (Figure 1h). The total score was 14.
Table
3: The development
of intraoperative scoring system.
|
Scoring
items |
0 |
1 |
2 |
3 |
|
Placental
location |
Non-anterior
|
Anterior
|
|
|
|
Relation
between placenta and cervix |
Low-lying
or partially covered |
Completely
covered |
|
|
|
The
invade depth of the muscle layer |
|
<1/2
|
≥1/2 |
Full
layer |
|
The
diameter of the invading area (cm) |
|
<3 |
3-5 |
>5 |
|
Neovascularization
density in the serosal surface of the lower segment of the uterus (area
method %) |
|
<30 |
30-50 |
>50 |
|
Invading
part |
Intrauterine
(cervix except) |
|
|
Cervical
and extrauterine * |
3.3. Evaluation of the Intraoperative Scoring Systems in 112 Patients with PAS
In total, 207 patients with PP were prospectively enrolled from April 2019 to March 2020. However, 112 cases of PAS were included in the study for the high-risk assessment. Maternal age, gestational age at delivery, gravidity, C-section history and maternal BMI are shown in (Table 4).
Table 4:
The relationship
between 6 scoring items and high-risk PAS and three important complications (hysterectomy,
DIC, and massive blood transfusion).
|
Scoring
items |
n (%)
|
X2
|
P |
n (%)
|
X2
|
P
|
n (%)
|
X2 |
P
|
n (%) |
X2 |
P |
|
High
risk PAS (n=52)
|
Hysterectomy
(n=11) |
DIC (n=22) |
Massive
blood transfusion (n=32) |
|||||||||
|
F1 |
50 (96.2%) |
21.779 |
<0.001 |
11 (100%) |
3.875 |
0.049 |
22 (100%) |
8.696 |
0.003 |
30 (93.8%) |
7.708 |
0.005 |
|
F2 |
51 (98.1%) |
6.837 |
<0.001 |
11 (100%) |
1.328 |
0.249 |
22 (100%) |
2.982 |
0.084 |
32 (100%) |
4.879 |
0.027 |
|
F3 |
30 (57.7%) |
31.590 |
<0.001 |
10 (90.9%) |
20.208 |
<0.001 |
14 (63.6%) |
13.366 |
<0.001 |
19 (59.4%) |
16.495 |
<0.001 |
|
F4 |
37 (71.2%) |
44.046 |
<0.001 |
10 (90.9%) |
14.223 |
<0.001 |
18 (81.8%) |
21.827 |
<0.001 |
23 (71.9%) |
21.234 |
<0.001 |
|
F5 |
41 (78.8%) |
63.689 |
<0.001 |
10 (90.9%) |
13.629 |
<0.001 |
22 (100%) |
42.311 |
<0.001 |
28 (87.5%) |
43.662 |
<0.001 |
|
F6 |
17 (32.7%) |
23.126 |
0.000 |
10 (90.9%) |
54.337 |
<0.001 |
13 (59.1%) |
41.005 |
<0.001 |
13 (40.6%) |
22.532 |
<0.001 |
F1: Anterior placenta; F2:
placenta completely covered; F3: placenta full layer invading; F4: The diameter
of the invading area>5cm; F5: Neovascularization density in the serosal
surface of the lower segment of the uterus (area >50%); F6: placenta
invading cervical or extrauterine (includes the bladder, rectum and vagina).
The chi-square test was used to examine the relationship between six parameters and high-risk PAS and severe complications, including hysterectomy, DIC and massive blood transfusion. Factors F1 to F6 had the highest scores among the items: F1, anterior placenta, 1 point; F2, placenta completely covered, 1 point; F3, placenta full-layer invasion, 3 points; F4, diameter of the invading area >5 cm, 3 points; F5, neovascularization density in the serosal surface of the lower segment of the uterus (area >50%), 3 points; and F6, placenta invading the cervical or extrauterine region (including the bladder, rectum and vagina), 3 points. All 6 factors were significantly related to high-risk PAS and massive blood transfusion (p<0.05). In addition to F2, other factors were significantly associated with hysterectomy and DIC (p<0.05) (Table 4).
3.4. The Cutoff Value of High-Risk PAS and its Severe Complications by the Intraoperative Scoring Systems in 112 Patients
The cutoff value was calculated to diagnose high-risk PAS and severe complications by the current intraoperative scoring system, as shown in (Table 5). The cutoff value for high-risk PAS was 6.5, with a Youden's index value of 0.792, sensitivity of 94.2% and specificity of 85%. The cutoff value for hysterectomy was 12.5, and the Youden's index value, sensitivity and specificity were 0.859, 90.9% and 95%, respectively. The cutoff value for DIC was 8.5, and the Youden's index value, sensitivity and specificity were 0.711, 95.5% and 75.6%, respectively. The cutoff value for massive blood transfusion was 8.5, and the Youden's index value, sensitivity and specificity were 0.876, 84.4% and 80%, respectively (Table 6).
Table
5: The cut-off value
and AUC of high-risk PAS and its sever complications.
|
|
cut-off
value |
Youden's
index |
sensitivity |
specificity |
AUC |
95%CI |
P
values |
|
High-risk
PAS |
6.5 |
0.792 |
0.942 |
0.850 |
0.939 |
0.891-0.987 |
<0.001 |
|
Hysterectomy |
12.5 |
0.859 |
0.909 |
0.950 |
0.945 |
0.885-1.000 |
<0.001 |
|
DIC |
8.5 |
0.711 |
0.955 |
0.756 |
0.918 |
0.866-0.971 |
<0.001 |
|
Massive
blood transfusion |
8.5 |
0.644 |
0.844 |
0.800 |
0.876 |
0.809-0.942 |
<0.001 |
Table
6: External validation
of the model on high risk of PAS, DIC and massive blood transfusion.
|
|
Kappa value |
P value |
95% CI |
Sensitivity |
Specificity |
PPV |
NPV |
|
High risk of PAS |
0.744 |
<0.001 |
0.595~0.892 |
90.0 % |
84.4 % |
90.0 % |
84.4% |
|
DIC |
0.230 |
0.001 |
0.069~0.390 |
100.0 % |
67.1 % |
19.4 % |
100.0% |
|
Massive blood transfusion |
0.481 |
<0.001 |
0.294~0.667 |
93.3 % |
74.6 % |
45.2 % |
98.0 % |
PPV: Positive Predictive
Value; NPV: Negative Predictive Value.
3.5. External Validation of the Scoring System about Higher Risk of PAS and its Severe Complications in 82 Patients
For high-risk PAS, the Kappa value was 0.744 (p<0.001), indicating substantial agreement, with a 95% confidence interval of 0.595 to 0.982. The sensitivity and positive predictive value (PPV) were both notably high at 90.0%, while specificity and negative predictive value (NPV) were both 84.4%. External validation data further supported the effectiveness of the scoring system, with only one case exceeding the threshold of 12.5. Remarkably, this patient, despite being identified as high risk, underwent a premature delivery without complications such as DIC, PPH and hysterectomy. As to DIC, the Kappa value was 0.230 (p=0.001), indicating fair agreement, with a 95% confidence interval of 0.069 to 0.390. The sensitivity and NPV were both excellent at 100.0%, but the specificity was modest at 67.1%, leading to a PPV of 19.4%. Regarding the prediction of massive blood transfusion, the scoring system demonstrated a Kappa value of 0.481 (p<0.001), with a 95% confidence interval of 0.294 to 0.667. High sensitivity (93.3%) and NPV (98.0%) were observed. However, the specificity was at 74.6%, and the PPV was at 45.2%.
4. Discussion
Prenatal diagnosis of PAS is paramount, as most patients with these disorders are asymptomatic. A precise diagnosis allows time for a multidisciplinary team to make adequate delivery plans, which will help decrease the risk of blood loss and massive blood transfusion, rate of hysterectomy, and even maternal deaths. Prenatal scoring systems developed to predict PAS severity were mainly based on imaging results, such as ultrasound and/or MRI [18-20]. Studies have reported that ultrasound findings of placental lacunae, obliteration of the uteroplacental demarcation, and PP are associated with the morbidly adherent placenta (MAP) and can be used for 'bedside' estimation of MAP risk when combined, yielding a better negative predictive value [19]. However, Weiniger et al. argued that combining some clinical features associated with placenta accreta through mathematical modeling has better positive predictive value than ultrasound findings alone [20]. Tanimura K et al. proposed a preoperative scoring system based on two categories: i) Past history of C-sections or uterine surgery and ii) Ultrasound and MRI findings. When the score was ≥8, they were viewed to be at high risk of PPH, and the use of internal iliac artery occlusion balloon catheters was suggested [21]. These previous reports have all demonstrated that the prenatal diagnosis scoring system is significantly helpful in reducing PAS-related blood loss and its complications.
However, prenatal scoring is not always accurate, with false-positive and false-negative possibilities. A false-positive diagnosis leads to a waste of medical resources, such as overuse of UAE or the balloon occlusion method, which can cause uterine necrosis [22, 23]. In this study, we found one case of uterine necrosis after UAE (0.89%). A false-negative diagnosis can lead to poor preparedness in terms of blood products and hemostatic measures, among other features. PAS is confirmed during surgery by clinical impressions or by pathological examination of the placenta and myometrium. The main goal of the current study was to propose a strategy for diagnosing high-risk PAS during surgery. The present system might be used as the final diagnostic criterion, which is more objective than solely the clinical impression or pathological examination.
In this analysis, we used 3 models of 3 independent high-risk factors related to massive PPH, and the maternal and newborn features were excluded, with only 3 placenta-related features remaining. Anterior placental and complete PP are two widely accepted independent risk factors for PPH and have been validated in many studies [24, 25]. Placental adhesion is commonly classified according to the depth of trophoblastic invasion into the myometrium, with the most severe type, placenta percreta, being associated with the highest risk of massive PPH, need for blood transfusion and admission to the ICU [26]. Higher neovascularization density in the serosal surface of the lower segment of the uterus indicates a high risk of PAS [27]. High-density vessels on the lower segment of the uterus were frequently noted during surgeries, especially in the case of placenta percreta, thereby increasing the opportunity for hysterectomy [28]. In the present system, placental adhesion features were divided into 4 items: the invading depth of the muscle layer, the diameter of the invading area, the invading part and neovascularization density. This was summarized by 8 experienced obstetrician experts who have performed more than 100 PAS surgeries after three Delphi rounds.
The relationship between the highest score among the 6 items and high-risk PAS was evaluated with the chi-square test. We verified that all 6 items were significantly related to the high-risk PAS diagnosis. When the score was 6.5, high-risk PAS could be diagnosed during surgery, yielding an area under the ROC curve of 0.939 (95% CI, 0.891-0.987), with a sensitivity of 94.2% and specificity of 85%. In addition to differentiating PAS severity, this system could also be used for the prediction of PAS-related complications, including hysterectomy, DIC and massive blood transfusion. If the score was ≥ 12.5, hysterectomy was likely, with an ROC value of 0.945 (95% CI, 0.885-1.000), sensitivity of 90.9% and specificity of 95%. Regarding the other two complications, their cutoff values were 8.5. The ROC curve for DIC was 0.918 (95% CI, 0.866-0.971), with a sensitivity of 95.5% and specificity of 75.6%. The ROC curve for massive blood transfusion was 0.876 (95% CI, 0.809-0.942), with a sensitivity of 84.4% and specificity of 80%. The evaluation of the scoring system using external data yielded promising results, with a relatively high Kappa value of 0.744 (95% CI, 0.595-0.982) for high-risk PAS.
Both sensitivity and PPV were notably high at 90.0%, while specificity and NPV also demonstrated relative strength at 84.4%. Regarding severe complications such as massive transfusions, the results indicated the system’s reliability in ruling out the need for such interventions (Kappa value of 0.481), with high sensitivity (93.3%) and NPV (98.0%), albeit with a moderate specificity (74.6%) and relatively low PPV (45.2%). However, in the assessment of DIC or hysterectomy, areas for further refinement and validation were identified. The present scoring system could be used to diagnose high-risk PAS as well as predict the possibility of serious complications. Especially during surgery, the score will help surgeons predict further complications and make appropriate decisions during surgery.
Our scoring system provides an objective strategy to quantitatively diagnose high-risk PAS, which is a good supplement for physiological examination and clinical impression. We provided a novel, easy, quick, and objective diagnostic method, which comprises a quantification of the physical examination and clinical impression, and an effective scoring system that can diagnose the severity of PAS and quickly predict its complications during surgery. It is important to acknowledge the limitations of this study, specifically that the scoring system was developed at a singular center though validated by internal and external data. To overcome this, a prospective, randomized, controlled, double-blind, multicenter trial should be conducted in the future.
5. Conclusion
Our system also provides a novel method to assess the possibility of PAS-related complications during surgery, which could help surgeons make quick decisions for the next step to avoid severe complications, such as DIC or even the death of the pregnant woman. Furthermore, the system could provide a basis for further adjustment of a more accurate preoperative scoring system in the future. All of these factors may ultimately improve the pregnancy outcomes of women at risk of such a potentially life-threatening obstetric disorder.
Data Availability
Ziyan Jiang and Zhiping Ge: Writing - original draft, manuscript writing, manuscript proofreading, formal analysis, satistical Analysis and data supervision. Qing Zuo and Shiyun Huang: Data collection, manuscript proofreading. Xinxin Zhu, Yihan Lu, Min Zhang, Yue Su, Lnahua Liu, Cen Cao, Haiyan Su, Junling Fei, Runrun Feng, Yufei Han, Xinmei Huang: Writing - review & editing and formal analysis. Ziyan Jiang, Zhiping Ge, Zijie Cheng and Hongmei Lu: Writing - review & editing, study design, literature review. Hongbin Xu, Lanhua Liu, Jinxia Xu: Data collection and analysis. All authors read and approved the final manuscript.
Conflicts of Interest
None.
Funding
The work was supported by Project of Jiangsu Health Commission (H2019008), National Natural Science Foundation of China (81971407), Natural Science Foundation of Jiangsu Province (BK20191070) and Postdoctoral Research Foundation of China (2021M691332).
Acknowledgments
We are grateful to all of the obstetrical experts engage in the development and evaluation of the intraoperative scoring system. Also, we thank all of the women who join in the study.
Ethical Approval
The retrospective and prospective studies were approved by the Ethical Board of our hospital (Ethics Committee 2020-SR-256 and 2019-SRFA-157, respectively).
REFERENCES
[1] Eric Jauniaux, Frederic Chantraine,
Robert M Silver, et al. “FIGO consensus guidelines on placenta accreta spectrum
disorders: Epidemiology.” Int J Gynaecol Obstet, vol. 140, no. 3, pp.
265-273, 2018. View at: Publisher
Site | PubMed
[2] Mulubrhan F Mogos, Jason L Salemi,
Mary Ashley, et al. “Recent trends in placenta accreta in the United States and
its impact on maternal-fetal morbidity and healthcare-associated costs,
1998-2011.” J Matern Fetal Neonatal Med, vol. 29, no. 7, pp. 1077-1082,
2016. View at: Publisher
Site | PubMed
[3] Jennifer C Hunt “Conservative
management of placenta accreta in a multiparous woman.” J Pregnancy,
vol. 2010, pp. 329618, 2010. View at: Publisher Site | PubMed
[4] Edwin Chandraharan, Archana Krishna
“Diagnosis and management of postpartum haemorrhage.” BMJ, vol. 358, pp.
j3875, 2017. View at: Publisher
Site | PubMed
[5] Nooraishah Yasin, Laura Slade, Elinor
Atkinson, et al. “The multidisciplinary management of placenta accreta spectrum
(PAS) within a single tertiary centre: A ten-year experience.” Aust N Z J
Obstet Gynaecol, vol. 59, no. 4, pp. 550-554, 2019. View at: Publisher Site | PubMed
[6] Daniela A Carusi “The Placenta
Accreta Spectrum: Epidemiology and Risk Factors.” Clin Obstet Gynecol,
vol. 61, no. 4, pp. 733-742, 2018. View at: Publisher Site | PubMed
[7] Eric Jauniaux, Amar Bhide “Prenatal
ultrasound diagnosis and outcome of placenta previa accreta after cesarean
delivery: a systematic review and meta-analysis.” Am J Obstet Gynecol,
vol. 217, no. 1, pp. 27-36, 2017. View at: Publisher Site | PubMed
[8] F D'Antonio, C Iacovella, J
Palacios-Jaraquemada, et al. “Prenatal identification of invasive placentation
using magnetic resonance imaging: systematic review and meta-analysis.” Ultrasound
Obstet Gynecol, vol. 44, no. 1, pp. 8-16, 2014. View at: Publisher Site | PubMed
[9] Christine H Comstock, Joseph J Love
Jr, Richard A Bronsteen, et al. “Sonographic detection of placenta accreta in
the second and third trimesters of pregnancy.” Am J Obstet Gynecol, vol.
190, no. 4, pp. 1135-1140, 2004. View at: Publisher Site | PubMed
[10] Valeria Romeo, Francesco Verde, Laura
Sarno, et al. “Prediction of placenta accreta spectrum in patients with
placenta previa using clinical risk factors, ultrasound and magnetic resonance
imaging findings.” Radiol Med, vol. 126, no. 9, pp. 1216-1225, 2021.
View at: Publisher
Site | PubMed
[11] Jennifer L Bailit, William A Grobman,
Madeline Murguia Rice, et al. “Morbidly adherent placenta treatments and
outcomes.” Obstet Gynecol, vol. 125, no. 3, pp. 683-689, 2015. View at: Publisher Site | PubMed
[12] K E Fitzpatrick, S Sellers, P Spark,
et al. “The management and outcomes of placenta accreta, increta, and percreta
in the UK: a population-based descriptive study.” BJOG, vol. 121, no. 1,
pp. 62-70, 2014. View at: Publisher
Site | PubMed
[13] M De Oliveira Carniello, L G Oliveira
Brito, L O Sarian, et al. “Diagnosis of placenta accreta spectrum in high-risk
women using ultrasonography or magnetic resonance imaging: systematic review
and meta-analysis.” Ultrasound Obstet Gynecol, vol. 59, no. 4, pp.
428-436, 2022. View at: Publisher
Site | PubMed
[14] A Bhide, N Sebire, A Abuhamad, et al.
“Morbidly adherent placenta: the need for standardization.” Ultrasound
Obstet Gynecol, vol. 49, no. 5, pp. 559-563, 2017. View at: Publisher Site | PubMed
[15] Marion Cortet, Delphine Maucort-Boulch,
Catherine Deneux-Tharaux, et al. “Severity of post-partum hemorrhage after
vaginal delivery is not predictable from clinical variables available at the
time post-partum hemorrhage is diagnosed.” J Obstet Gynaecol Res, vol.
41, no. 2, pp. 199-206, 2015. View at: Publisher Site | PubMed
[16] Stephanie A Savage, Joshua J
Sumislawski, Ben L Zarzaur, et al. “The new metric to define large-volume
hemorrhage: results of a prospective study of the critical administration
threshold.” J Trauma Acute Care Surg, vol. 78, no. 2, pp. 224-229, 2015.
View at: Publisher
Site | PubMed
[17] Riaz Agha, Ali Abdall-Razak, Eleanor
Crossley, et al. “STROCSS 2019 Guideline: Strengthening the reporting of cohort
studies in surgery.” Int J Surg, vol. 72, pp. 156-165, 2019. View at: Publisher Site | PubMed
[18] Yoshiko Ueno, Tetsuo Maeda, Utaru
Tanaka, et al. “Evaluation of interobserver variability and diagnostic
performance of developed MRI-based radiological scoring system for invasive
placenta previa.” J Magn Reson Imaging, vol. 44, no. 3, pp. 573-583,
2016. View at: Publisher
Site | PubMed
[19] R Maymon, Y Melcer, M Pekar-Zlotin,
et al. “Bedside risk estimation of morbidly adherent placenta using simple
calculator.” Arch Gynecol Obstet, vol. 297, no. 3, pp. 631-635, 2018.
View at: Publisher
Site | PubMed
[20] C F Weiniger, S Einav, L Deutsch, et
al. “Outcomes of prospectively-collected consecutive cases of
antenatal-suspected placenta accreta.” Int J Obstet Anesth, vol. 22, no.
4, pp. 273-279, 2013. View at: Publisher Site | PubMed
[21] Kenji Tanimura, Mayumi Morizane,
Masashi Deguchi, et al. “A novel scoring system for predicting adherent
placenta in women with placenta previa.” Placenta, vol. 64, no. 27-33,
2018. View at: Publisher
Site | PubMed
[22] Esther Ruiz Sánchez, Javier Peinado
Rodenas, Leyre Gil Martínez-Acacio, et al. “Uterine necrosis. A rare
complication of embolisation due to post-partum haemorrhage.” J Gynecol
Obstet Hum Reprod, vol. 50, no. 2, pp. 101773, 2021. View at: Publisher Site | PubMed
[23] Devin D Smith, Annette Perez-Delboy,
William M Burke, et al. “Buttock Necrosis after Uterine Artery Embolization for
Delayed Hysterectomy in Placenta Percreta.” Case Rep Obstet Gynecol,
vol. 2016, pp. 6921280, 2016. View at: Publisher Site | PubMed
[24] Michaela Granfors, Olof Stephansson,
Margit Endler, et al. “Placental location and pregnancy outcomes in nulliparous
women: A population-based cohort study.” Acta Obstet Gynecol Scand, vol.
98, no. 8, pp. 988-996, 2019. View at: Publisher Site | PubMed
[25] Ji Yeon Lee, Eun Hee Ahn, Sukho Kang,
et al. “Scoring model to predict massive post-partum bleeding in pregnancies
with placenta previa: A retrospective cohort study.” J Obstet Gynaecol Res,
vol. 44, no. 1, pp. 54-60, 2018. View at: Publisher Site | PubMed
[26] Louis Marcellin, Pierre Delorme,
Marie Pierre Bonnet, et al. “Placenta percreta is associated with more frequent
severe maternal morbidity than placenta accreta.” Am J Obstet Gynecol,
vol. 219, no. 2, pp. 193.e1-193.e9, 2018. View at: Publisher Site | PubMed
[27] Giuseppe Calì, Francesco D'Antonio, Francesco Forlani, et al. “Ultrasound Detection of Bladder-Uterovaginal Anastomoses in Morbidly Adherent Placenta.” Fetal Diagn Ther, vol. 41, no. 3, pp. 239-240, 2017. View at: Publisher Site | PubMed
[28] Ahmed M Hussein, Karin Fox, Amar Bhide, et al. “The impact of preoperative ultrasound and intraoperative findings on surgical outcomes in patients at high risk of placenta accreta spectrum.” BJOG, vol. 130, no. 1, pp. 42-50, 2023. View at: Publisher Site | PubMed
