Received: Fri 09, Aug 2024
Accepted: Thu 12, Sep 2024
1. Introduction
Aggressive malignant esophageal cancer is one of the leading causes of cancer-related mortality globally (Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries) [1]. There are two major histologic types of esophageal cancer (EC), which is squamous cell carcinoma and adenocarcinoma. Esophageal squamous cell carcinoma (ESCC) is the more common histologic type in east Asia [2].
According to the National Comprehensive Cancer Network guidelines (Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology), neoadjuvant chemotherapy or chemoradiotherapy is strongly recommended for patients with locally advanced esophageal cancer. However, the 5-year overall survival rate of approximately 20% after this neoadjuvant therapy is obviously unsatisfactory [3]. In recent years, the use of immune checkpoint inhibitors has become an important regulatory factor in cancer intervention. Immunotherapy prolonged OS versus chemotherapy when used as the second-line treatment for advanced EC, with manageable toxicity [4]. Several PD-1 or PD-L1 inhibitors have shown clinical effects in the treatment of ESCC, such as in patients with metastatic esophageal cancer, pembrolizumab combined with chemotherapy significantly prolonged survival compared with chemotherapy alone [5]. Recently, some scholars reported that nivolumab and camrelizumab, respectively, combined with first-line chemotherapy drugs in the treatment of ESCC, played a synergistic role in reducing tumor burden [6, 7].
Durvalumab, as a PD-L1 inhibitor, has achieved good therapeutic effects as a single therapy or dual immunotherapy for several types of cancer [8-10]. In addition, in non-small cell lung cancer patients, durvalumab combined with chemotherapy is associated with higher survival rates compared to chemotherapy alone [11-13]. Furthermore, in resectable locally advanced head and neck squamous cell carcinoma, the phase 2 study of neoadjuvant chemotherapy plus durvalumab showed encouraging rates of pathologic complete response and clinical pathologic downstaging [14]. Therefore, our study compared the efficacy and safety between durvalumab combined with chemotherapy and chemotherapy alone in neoadjuvant therapy in ESCC patients.
2. Methods
2.1. Participants
This single center, prospective, single-arm phase II study was done at Peking Union Medical College Hospital (PUMCH, Beijing, China), from October 2020 to October 2023. Correspondingly, all patients undergoing neoadjuvant chemotherapy alone followed by esophagectomy during the same period were retrospective analyzed as the control group.
Inclusion criteria: Patients had locally advanced ESCC histologically diagnosed by endoscopic biopsy with either T1-2, N(+)M0 or T3-T4a N(any)M0 clinical stage according to the American Joint Committee on Cancer (AJCC). Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Exclusion criteria: Patients had pathological types of adenocarcinoma or other histologic types; patients had a time interval between the last medication and surgery exceeding three months; patients had surgical contraindications or determined as inoperable cases by physician after neoadjuvant therapy and systematic evaluation. Tumor staging before enrollment was evaluated by upper gastrointestinal endoscopy; endoscopic ultrasound (EUS); enhanced computed tomography (CT) of the neck, thorax, and abdomen; and positron emission tomography with integrated CT (PET-CT). Cervical ultrasound examination is also performed to assess the extent of involvement of lymph nodes in neck and supra-clavicular regions. Lymph node stations were defined according to the Japanese Classification of Esophageal Cancer (11th edition). The PUMCH ethics committee approved the protocol and all amendments. All patients provided written informed consent. The study is registered at clinical trial (NCT04568200).
2.2. Neoadjuvant Therapy
In the chemotherapy group, patients received 2-4 cycles of cisplatin (75 mg/m2 of body-surface area, D1) and paclitaxel (175 mg/m2 body-surface area, D1) via IV every 3 weeks. In the chemoimmunotherapy group, patients received 4 cycles of durvalumab (1500mg D1), carboplatin (AUC=5, D1) and docetaxel (75 mg/m2 of body-surface area, D1) via IV every 3 weeks. The baseline assessments by EUS, enhanced CT, and PET-CT were performed within 28 days before the neoadjuvant treatment began. The patients were reevaluated by enhanced CT of the chest and upper abdomen after the neoadjuvant therapy was completed. If there was no evidence of metastatic disease, the McKeown esophagectomy was performed within 4-8 weeks.
2.3. Surgery
The surgery was performed using a standard minimally invasive McKeown’s procedure and systemic lymph node dissection by qualified thoracic surgeons. After dissection of the thoracic esophagus and proximal stomach, a tubular gastroplasty was performed, and a hand-sewn esophagogastric anastomosis was performed in the neck. Jejunostomy and jejunal tube placement were routinely performed during the operation.
2.4. Pathological Response
Pathological response was evaluated by the College of American Pathologists (CAP) protocol. (Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 47, 141-146 (2005)). Programmed death ligand 1 (PD-L1) expression was evaluated using immunohistochemical staining with the 22C3 antibody (Dako, Glostrup, Denmark). Both combined positive score (CPS) and tumor proportion score (TPS) were calculated. CPS was defined as the number of PD-L1-positive cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells multiplied by 100. TPS was determined as the percentage of tumor cells with partial or complete staining relative to all tumor cells in the sample.
2.5. Follow-up Evaluation
The chest X-ray was performed on postoperative day (POD) 1. The patients received full parenteral nutrition starting from POD 1 to POD 3. On POD 3, patients started using enteral nutrition through jejunal feeding tubes and gradually reduced the usage of parenteral nutrition. On POD 5 or 6, the patient would be discharged when they could fully rely on jejunal nutrition support. The patients were required to continue the strict prohibition of oral intake of food or water for the following 3 weeks after surgery.
After an upper gastrointestinal contrast was performed to confirm there’s no leakage of the anastomosis or the gastric tube, the patient would begin liquid diet and transition gradually to normal diet. and underwent the adjuvant therapy according to the postoperative pathology. The next follow-up visits were scheduled at 4 months after operation and every 4 months thereafter. The serum tumor markers, cervical lymph node ultrasound and enhanced CT scan of neck, chest and abdomen were performed at each follow-up visit.
3. Results
3.1. Baseline Demographics
From October 2020 to December 2023, 27 patients were enrolled in the study and received 4 cycles of the neoadjuvant therapy. Twenty patients underwent surgery after the neoadjuvant therapy. 85.2% patients were male sex (23/27). The median age of the patients was XX years. The ECOG performance status of 96.3% patients was 1. More than half of the patients had smoking (16/27, 59.3%) or drinking history (16/27, 59.3%). Nine patients had family history of cancer. Most of the tumors were in the middle (12/27, 44.4%) or distal (11/27, 40.7%) third of the esophagus. Most of the patients had more invasive disease, with 9 having stage T3 (33.3%) and 17 having stage T4 (63%). The detailed baseline characteristics are shown in (Table 1). Forty patients in total underwent the neoadjuvant chemotherapy alone followed by esophagectomy were analyzed as the control group. There was no significant difference in gender, age, tumor location, the stage before chemotherapy, tumor length, and PD-L1 expression between the two groups.
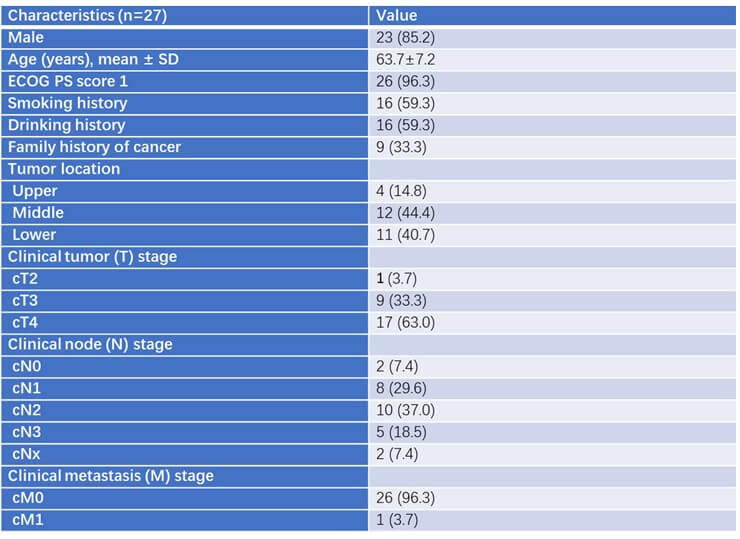
3.2. Treatment Outcomes
The flowchart describing enrollment of patients is shown in (Figure 1). After 4 cycles of chemoimmunotherapy, 4 patients refused to undergo the surgery because of personal choice and 3 patients had disease progression. Therefore, 20 patients underwent the standard McKeown’s procedure and systemic lymph node dissection.
3.3. Safety
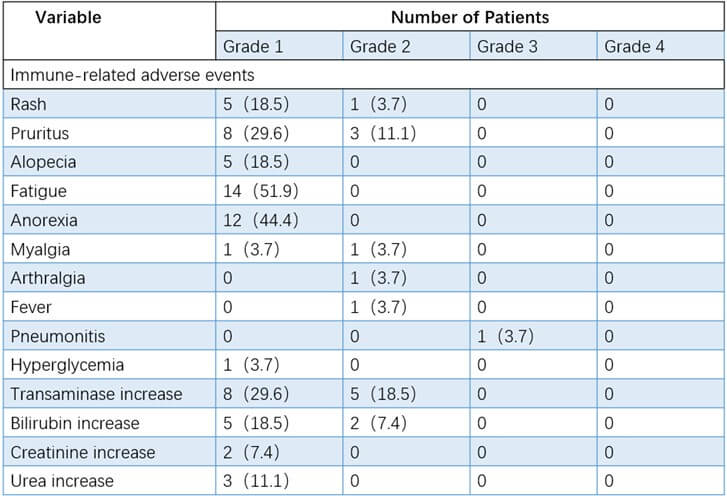
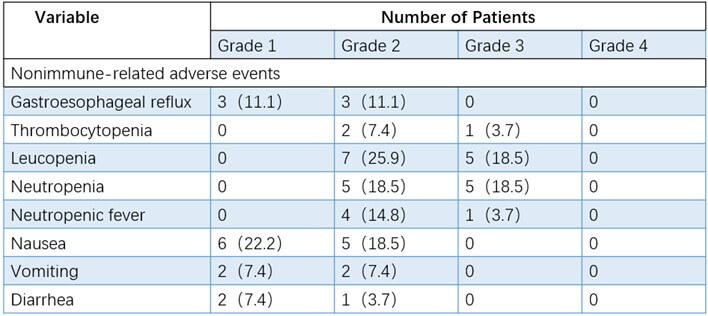
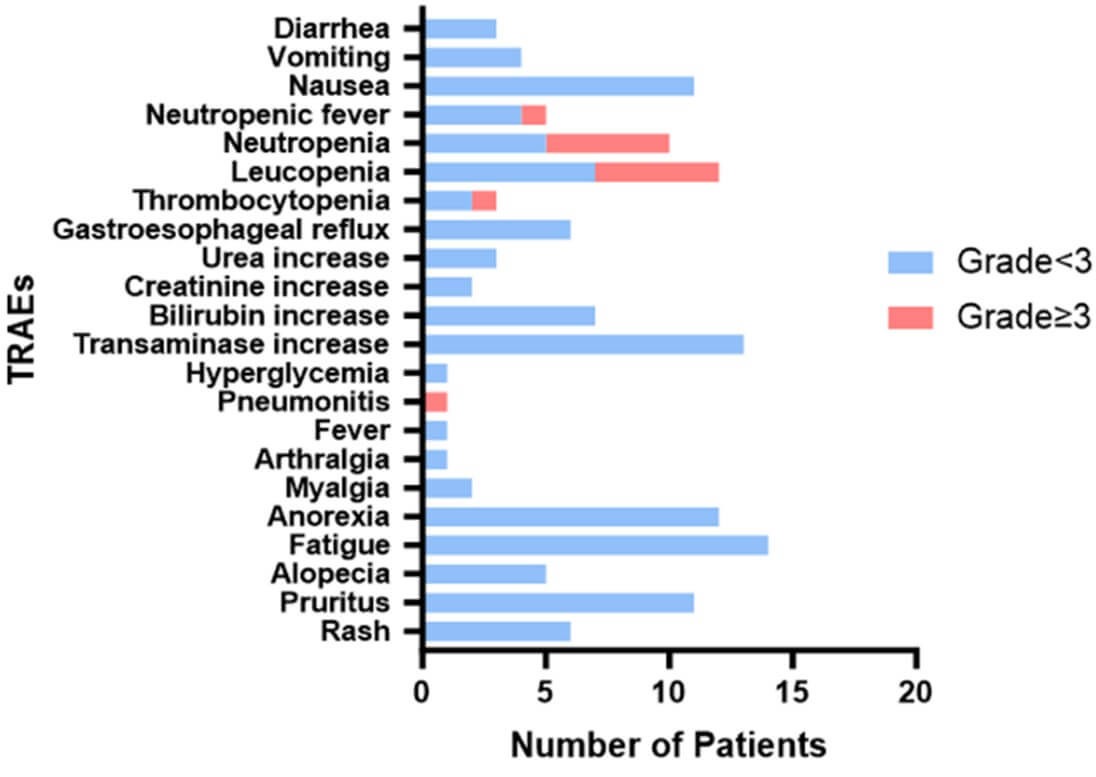
In this phase 2 study of durvalumab, the overall immunotoxicity was manageable. As shown in (Table 2), the most common immune-related adverse events (iRAEs) were anorexia (12/27, 44.4%) and fatigue (14/27, 51.9%). Only one patient (3.70%) required further hospitalization due to pneumonia. Among the nonimmune-related adverse events of neoadjuvant therapy, the most common symptoms were leukopenia (44.44%), nausea (40.74%), and neutropenia (37.04%, (Table 3)). Grade 3-4 nonimmune-related adverse events were reported in 12 patients (leukopenia [5/27, 18.5%], thrombocytopenia [5/27, 18.5%], neutropenia [1/27, 3.7%] and neutropenic fever [1/27, 3.7%]. No treatment-related surgical delay or death was observed. Some patients received further intervention before undergoing surgery according to the grades of adverse events (Figure 2). All patients with adverse events in this study were managed well without sequelae up to now.
3.4. Efficacy
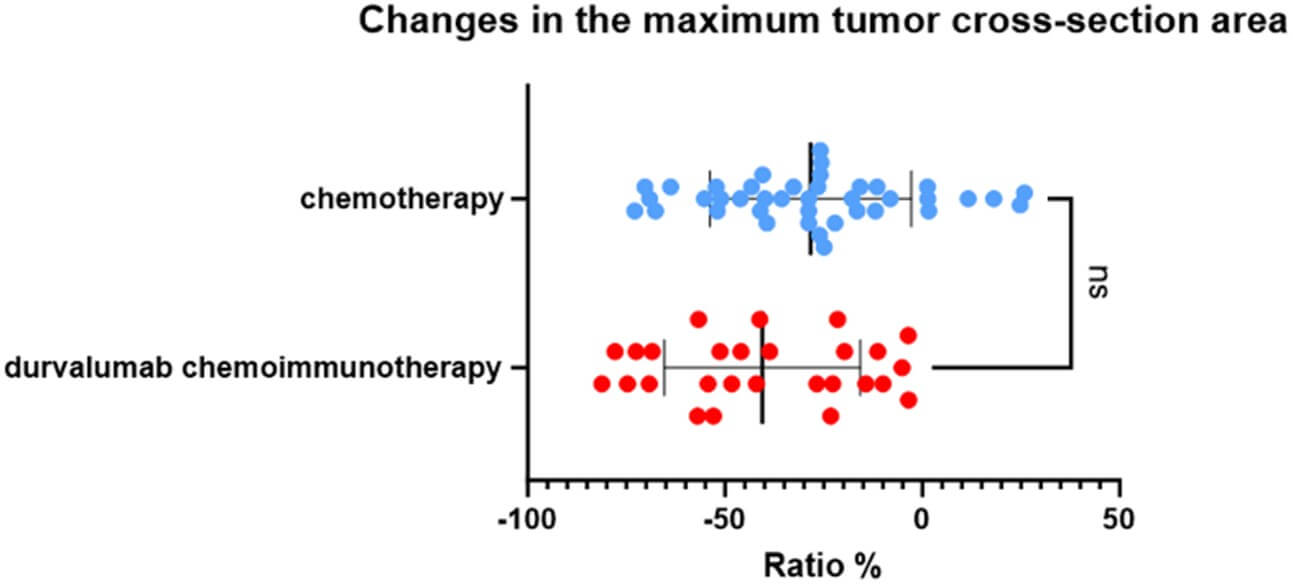
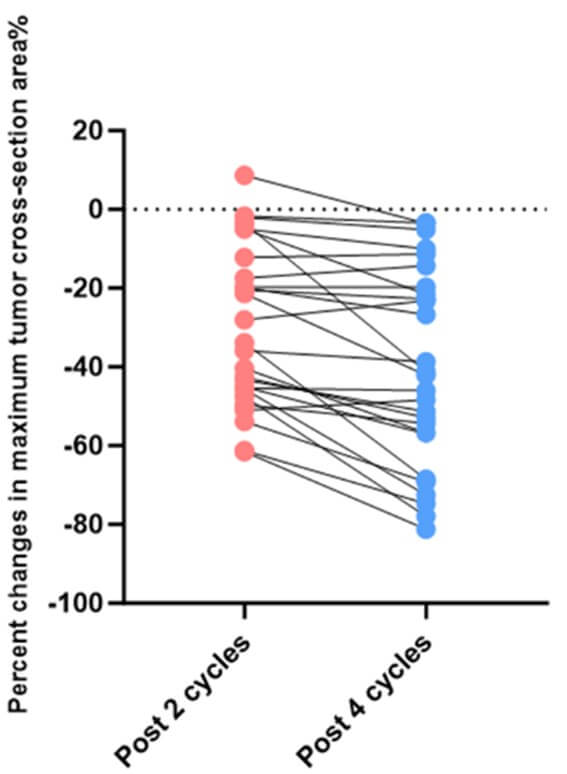
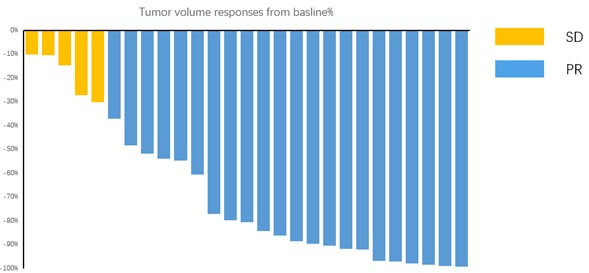
According to the RECIST 1.1 criteria, 27 patients who underwent 4 cycles of neoadjuvant chemoimmunotherapy imaging attained an objective response (Figure 5): Twenty-two (81.48%) patients achieved partial response (PR), while the other 5 (18.52%) patients had stable disease (SD). Only 27 of 40 patients in the chemotherapy alone group achieved PR, respectively. There was no significant difference in clinical response rates between the two groups (p=0.205). Furthermore, the average reduction in the maximum tumor cross-section area in the chemoimmunotherapy group was 40.53%. On the other hand, the average reduction in the maximum cross-section area of the target lesions in patients receiving neoadjuvant chemotherapy alone was 28.32% (Figure 3).Also there was no significant difference between the two groups (P=0.0562). The maximum tumor cross-section area of most patients decreased further after 4 cycles of treatment compared to their statistics after 2 cycles of treatment (Table 4).
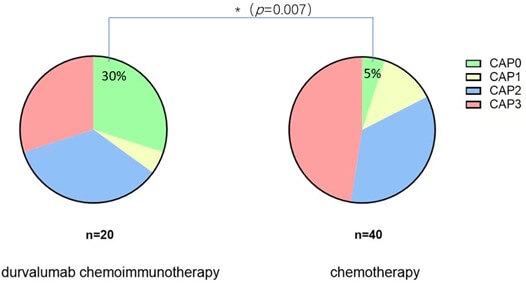
As for the pathological response, the pCR (CAP 0) was observed in 22.2% (6/27) patients in the chemoimmunotherapy group, while only 5% (2/40) patients achieved pCR in chemotherapy alone group. There was a significant difference in the pCR rate between the two groups ((Figure 6), p=0.007).
3.5. Surgical Outcomes and Perioperative Complications
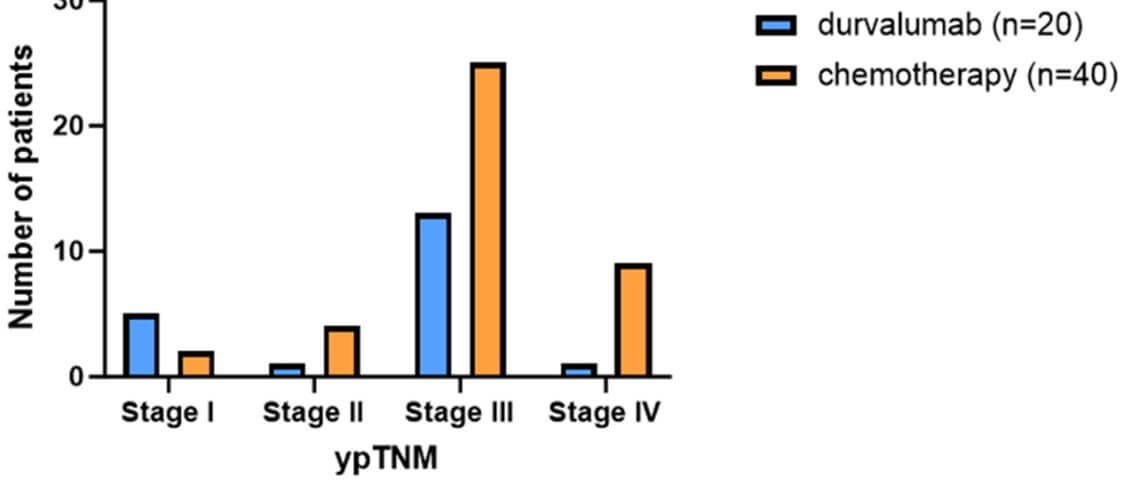
In the post-operative TNM pathological staging, only 1 (5%) patient in the group treated with neoadjuvant chemotherapy combined with durvalumab reached stage 4, while the number of patients in the control group of neoadjuvant chemotherapy alone was 9 (22.5%, P=0.086, (Figure 7)).
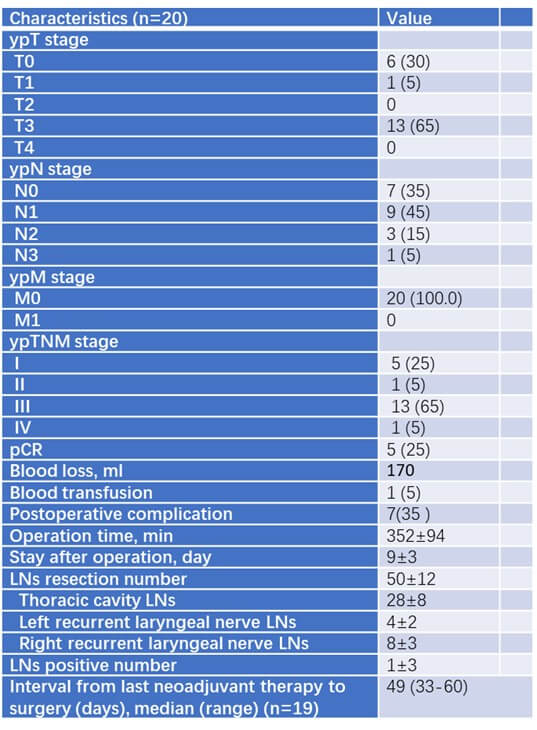
In the chemoimmunotherapy group, 20 patients underwent esophagectomy performed by experienced thoracic surgeons (Table 4). All patients underwent R0 resection. The median blood loss was 170 ml and only 1 patient required blood transfusion during the surgery. The median operation time was 352 minutes. Perioperative complications were manageable (7/20, 35%). The median postoperative hospital stay was 9 days. The median number of resected lymph nodes was 50. The median interval time from last neoadjuvant therapy to surgery was 49 days.
4. Discussion
Our study shows that durvalumab combined with neoadjuvant chemotherapy can more effectively reduce the volume of target lesions (although limited by a small sample size, there is no significant difference) and increase the resectability and negative resection margin rate compared to neoadjuvant chemotherapy alone. And compared to other neoadjuvant immunotherapy drugs such as Pembrolizumab, durvalumab-induced immune-related adverse events are relatively mild. Therefore, in clinical applications, durvalumab is more recommended for patients with high ECOG scores, older age, and more comorbidities. At the same time, for the ratio of postoperative pathological regression, durvalumab combined with neoadjuvant chemotherapy can significantly increase the proportion of pCR and predictively improve patient’s prognosis.
There are some limitations in our study. First, this is a non-randomized study. The analysis of the efficacy of a single center, single immunotherapy drug combined with neoadjuvant chemotherapy may be affected by factors such as tumor location, patient age, and severity of comorbidities, resulting in deviations in study results and follow-up data due to the small sample size. Secondly, the patients who received neoadjuvant chemotherapy alone generally chose the TP regimen (cisplatin + paclitaxel), while the patients who received neoadjuvant chemotherapy combined with immunotherapy used the DC regimen (carboplatin + docetaxel). The increase in variables will decrease the accuracy of evaluating the effectiveness of treatment. Finally, we have not conducted a horizontal comparison of the effectiveness of durvalumab with other immunotherapeutic drugs in terms of pathological response rate, so it is inappropriate to determine whether durvalumab has any advantages over other immunotherapeutic drugs used in locally advanced esophageal cancer.
5. Conclusion
Durvalumab combined with neoadjuvant chemotherapy shows gratifying safety and feasibility in phase 2 clinical study. And it has indeed played a positive role in reducing tumor burden and improving pathological response rate. However, the impact on post-operation complication, as well as the survival and recurrence time of patients treated with durvalumab combined with neoadjuvant chemotherapy, require more follow-up data for evaluation and summary.
REFERENCES
[1] Lindsey A Torre, Freddie Bray,
Rebecca L Siegel, et al. “Global cancer statistics, 2012.” CA Cancer J Clin,
vol. 65, no. 2, pp. 87-108, 2015. View at: Publisher Site | PubMed
[2] Mahdi Sheikh, Gholamreza Roshandel,
Valerie McCormack, et al. “Current Status and Future Prospects for Esophageal
Cancer.” Cancers (Basel), vol. 15, no. 3, pp. 765, 2023. View at: Publisher Site | PubMed
[3] Masanori Ochi, Yuji Murakami, Ikuno
Nishibuchi, et al. “Long-term results of definitive chemoradiotherapy for
unresectable locally advanced esophageal squamous cell carcinoma.” J Radiat
Res, vol. 62, no. 1, pp. 142-148, 2021. View at: Publisher Site | PubMed
[4] Takashi Kojima, Manish A Shah, Kei
Muro, et al. “Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus
Chemotherapy in Advanced Esophageal Cancer.” J Clin Oncol, vol. 38, no.
35, pp. 4138-4148, 2020. View at: Publisher
Site | PubMed
[5] Shun Yamamoto, Ken Kato
“Pembrolizumab for the treatment of esophageal cancer.” Expert Opin Biol
Ther, vol. 20, no. 10, pp. 1143-1150, 2020. View at: Publisher Site | PubMed
[6] “Errata.” J Clin Oncol, vol.
39, no. 35, pp. 4000, 2021. View at: Publisher Site | PubMed
[7] Ken Kato, Byoung Chul Cho, Masanobu
Takahashi, et al. “Nivolumab versus chemotherapy in patients with advanced
oesophageal squamous cell carcinoma refractory or intolerant to previous
chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3
trial.” Lancet Oncol, vol. 20, no. 11, pp. 1506-1517, 2019. View at: Publisher Site | PubMed
[8] Marion Thibaudin, Jean-David Fumet,
Benoist Chibaudel, et al. “First-line durvalumab and tremelimumab with
chemotherapy in RAS-mutated metastatic colorectal cancer: a phase 1b/2 trial.” Nat
Med, vol. 29, no. 8, pp. 2087-2098, 2023. View at: Publisher Site | PubMed
[9] Yelena Y Janjigian, Eric Van Cutsem,
Kei Muro, et al. “MATTERHORN: phase III study of durvalumab plus FLOT
chemotherapy in resectable gastric/gastroesophageal junction cancer.” Future
Oncol, vol. 18, no. 20, pp. 2465-2473, 2022. View at: Publisher Site | PubMed
[10] Tiago Biachi de Castria, Danny N
Khalil, James J Harding, et al. “Tremelimumab and durvalumab in the treatment
of unresectable, advanced hepatocellular carcinoma.” Future Oncol, vol.
18, no. 33, pp. 3769-3782, 2022. View at: Publisher Site | PubMed
[11] “Durvalumab Extends OS in NSCLC.” Cancer
Discov, vol. 8, no. 12, pp. Of5, 2018. View at: Publisher Site | PubMed
[12] Scott J Antonia, Augusto Villegas,
Davey Daniel, et al. “Overall Survival with Durvalumab after Chemoradiotherapy
in Stage III NSCLC.” N Engl J Med, vol. 379, no. 24, pp. 2342-2350,
2018. View at: Publisher
Site | PubMed
[13] Young-Hak Kim “Durvalumab after
Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer.” N Engl J Med,
vol. 380, no. 10, pp. 989-990, 2019. View at: Publisher Site | PubMed
[14] R L Ferris, R Haddad, C Even, et al. “Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study.” Ann Oncol, vol. 31, no. 7, pp. 942-950, 2020. View at: Publisher Site | PubMed
