Received: Thu 31, Jul 2025
Accepted: Thu 28, Aug 2025
Abstract
Background: Breast cancer remains the leading malignancy in women worldwide, with de-escalating neoadjuvant therapy being a key research focus. This study evaluated the prognostic value of MRI-detected complete response (CR) during neoadjuvant chemotherapy (NAT) across molecular subtypes, aiming to identify candidates for treatment de-escalation.
Methods: In this multicenter retrospective study (n=1,160 stage II-III patients), patients underwent bimonthly DCE-MRI assessments during 6 cycles of NAT followed by surgery. The primary endpoints were 8-year disease-free survival (DFS), analyzed using Kaplan-Meier curves and log-rank tests. Secondary endpoints included pathological complete response (pCR) rates in patients with radiological CR at 2, 4, or 6 cycles, stratified by subtype.
Results: In HR-/HER2+ patients, early radiological CR (≤4 cycles) was associated with superior DFS compared to late CR (8-year DFS: 2 vs. 6 cycles: P = 0.013; 4 vs. 6 cycles: P = 0.037). Other subtypes demonstrated similar trends for DFS. HR-/HER2- patients achieving radiological CR at 2 cycles had better outcomes than those with late CR (8-year DFS: 2 vs. 6 cycles: P = 0.048). The pCR rate was highest in HR-/HER2+ patients.
Conclusion: DCE-MRI-based early CR assessment predicts long-term survival and may guide NAT de-escalation, though subtype-specific variations warrant complementary biomarkers.
Keywords
Breast cancer, neoadjuvant chemotherapy (NAT), dynamic contrast-enhanced MRI (DCE-MRI), radiological complete response, pathological complete response (pCR), prognosis
1. Introduction
Breast cancer remains the most prevalent malignancy in women, with approximately 30-40% of patients presenting as locally advanced (stage II-III) disease at diagnosis [1, 2]. Pathology complete response (pCR) after neoadjuvant chemotherapy (NAT) is a well-defined prognostic indicator. The 5-year disease-free survival rate of patients who achieve pCR is 15-20% higher than that of patients without pCR [3]. However, the assessment of pCR is invasive and cannot monitor treatment efficacy in real time.
MRI has emerged as a cornerstone for monitoring NAT response in breast cancer, offering superior soft-tissue resolution and functional assessment of tumor vasculature compared to conventional imaging [4]. By providing quantitative functional insights into pathophysiological processes, DCE-MRI complements conventional MRI, addressing its limitations in functional tissue characterization [5]. By tracking gadolinium-based contrast kinetics, dynamic contrast-enhanced MRI (DCE-MRI) quantifies tissue perfusion and vascular permeability, enabling early detection of biological changes predictive of treatment efficacy [6-8]. Research by Takayo et al. demonstrated that DCE-MRI is highly accurate and has good efficacy in the treatment of neoadjuvant chemo-cancer, reaching up to 88.7% [9]. Furthermore, other research findings reported 83-94% accuracy in pathological response prediction, with subtype-specific variations noted.
Currently, there is a trend toward de-escalation in the treatment of breast cancer. Both surgical procedures and NAT may reduce treatment-related side effects while maintaining therapeutic efficacy. As a result, numerous studies in this regard have emerged. For example, the study by Henry et al. explored the possibility of omitting surgery after NAT [10]. The TRAIN-3 study has provided important insights into the predictive value of early radiological CR in HER2-positive breast cancer patients [11]. Early radiological CR can identify a subgroup of patients who are highly sensitive to treatment, and the feasibility of omitting some cycles of NAT has been explored. While the TRAIN-3 study relied on conventional MRI, our utilization of DCE-MRI yields greater sensitivity in identifying breast cancer imaging modifications. This multicenter real-world study aimed to determine the prognostic value of CR timing on long-term survival (DFS) and its correlation with pCR, addressing a critical gap in personalized NAT strategies.
2. Methods and Materials
2.1. Study Design and Participants
This is a multicenter retrospective study conducted at three hospitals in China, namely, the First Hospital of China Medical University, the Affiliated Zhongshan Hospital of Dalian University, and the First Affiliated Hospital of Jinzhou Medical University. This study included 1,527 patients aged 22-82 years with stage II-III breast cancer (2016-2023). Hormone receptor (HR) and HER2 status were defined per ASCO/CAP guidelines. All patients had undergone computed tomography (CT) scans of the head, lungs, and abdomen to exclude distant metastases. A total of 367 patients were excluded on the basis of the following criteria: incomplete completion of six cycles of NAT (n = 94); absence of surgical treatment (n = 52); male breast cancer (n = 2); gestational breast cancer (n = 3); bilateral breast cancer (n = 17); a prior diagnosis of other malignant tumors (n = 38); incomplete follow-up data, including follow-up durations shorter than one year (n = 83); and missing clinical and pathological data (n = 78) (Figure 1).
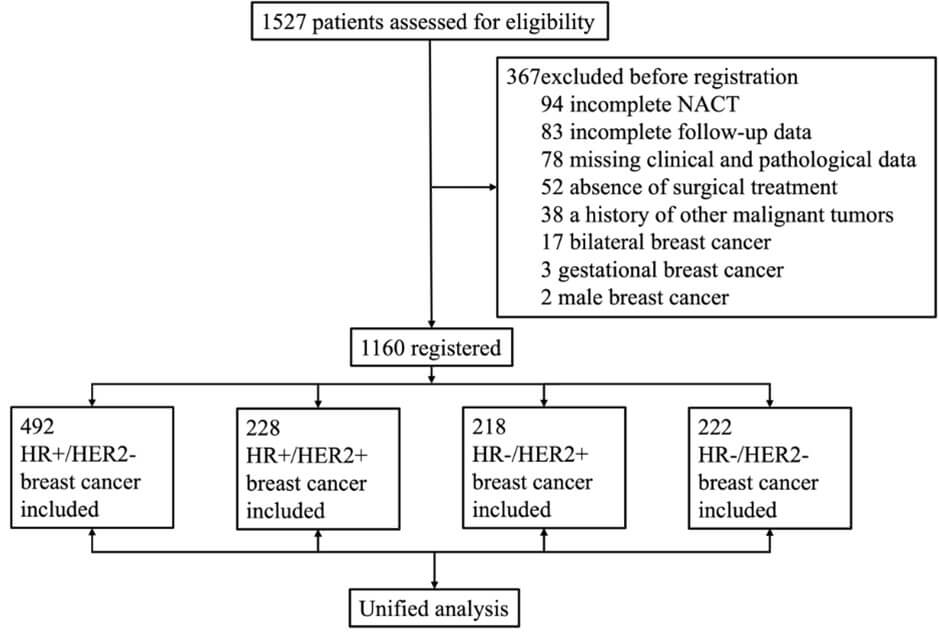
All patients underwent breast DCE-MRI as well as ultrasound examinations of the axillary and clavicular regions. In cases where the lymph nodes were positive (characterized by a short axis of 10 mm or greater or cortical thickness), a fine-pi-needle was employed for diagnostic confirmation. Prior to the commitment of NAT, markers were implanted at the site of the primary breast tumor for the purpose of positioning. The research protocol was approved by the Medical Ethics Committee of the First Hospital of China Medical University. This retrospective study was granted a waiver of informed consent by the Ethics Committee in accordance with applicable regulations.
2.2. Procedures
All patients received NAT once every three weeks. For patients with hormone receptor-positive and HER2-positive (HR+/HER2+) as well as hormone receptor-negative and HER2-positive (HR-/HER2+) breast cancer, those who presented before June 2020 received either paclitaxel (175 mg/m², on day 1 of each 3-week cycle), carboplatin (area under the concentration time curve of 6 mg/min/mL, on day 1 of each cycle), and trastuzumab (loading dose of 8 mg/kg on day 1 of cycle 1, followed by 6 mg/kg on day 1 of subsequent cycles), or paclitaxel, cyclophosphamide (600 mg/m²), and trastuzumab using the same trastuzumab dosing schedule. After June 2020, these patients were treated with a four-drug regimen consisting of paclitaxel, carboplatin, trastuzumab, and pertuzumab (loading dose of 840 mg on day 1 of cycle 1, followed by 420 mg on day 1 of subsequent cycles), which was administered intravenously for six cycles. Subcutaneous administration of trastuzumab (600 mg) was permitted. Among patients with hormone receptor-positive and HER2-negative (HR+/HER2-) breast cancer, 318 patients (65%) received the 6CEX regimen, which included cyclophosphamide (600 mg/m²), epirubicin (90-100 mg/m²), and capecitabine (2,500 mg/m²/day), all of which were administered on day 1 of each 3-week cycle. The remaining 35% of HR+/HER2- patients received the same regimen as those with hormone receptor-negative and HER2-negative (HR-/HER2-) breast cancer, which comprised paclitaxel (175 mg/m²), epirubicin (90-100 mg/m²), and cyclophosphamide (600 mg/m²), which were administered every 3 weeks for six cycles.
During the neoadjuvant treatment, the same methods used in the baseline assessment (i.e., breast ultrasound and DCE-MRI) were employed. The response of the tumor to treatment was monitored via DCE-MRI every two treatment cycles. There was no contrast enhancement in the primary tumor area, although complete disappearance of the tumor was not needed. If minimal contrast enhancement similar to (or less than) that of the surrounding or contralateral normal breast tissue was observed in the primary tumor area, it was considered physiological. Residual small lesions in the original tumor bed with pathological enhancement were not regarded as a radiological CR. The DCE-MRI response assessment of all patients was uniformly re-evaluated by three professional radiologists. If all three radiologists agreed that a patient achieved a radiological CR, the patient was determined to have a radiological CR. In the case of any dissenting opinions, the case was referred to a radiologist with more experience who operated the imaging equipment for review.
Patients underwent breast-conserving surgery or mastectomy within four weeks after the last chemotherapy. Axillary surgical procedures included sentinel lymph node surgery, axillary lymph node dissection, or a combination of both. For HER2-positive patients who achieved a pCR, adjuvant treatment with trastuzumab and pertuzumab for one year was completed without additional chemotherapy. Clinicians determined whether all patients should receive radiotherapy and endocrine therapy according to the patients' specific conditions and in line with the guidelines.
2.3. Outcomes
The primary endpoints of this study was 8-year DFS. DFS was defined as the time elapsed from patient enrolment until disease recurrence or death (from any cause). The key secondary endpoint was pCR, which was defined as the absence of invasive tumor cells in the breast and axilla upon sufficient pathological sampling after surgery, regardless of the presence of in situ lesions. A radiological CR was defined as the absence of pathological enhancement in the original tumor area on breast DCE-MRI.
2.4. Statistical Analysis
Primary endpoints (8-year DFS) were analyzed using Kaplan-Meier curves with log-rank tests, stratified by CR timing (2/4/6 cycles) and subtype. The 95% confidence intervals (CIs) of DFS was estimated via the hazard model. In the Kaplan-Meier survival curves, '2-cycle CR' refers to patients who achieved radiological CR during the first two cycles of NAT. '4-cycle CR' denotes patients who attained radiological CR in cycles 3 or 4 (excluding those who had already achieved 2-cycle CR). '6-cycle CR' represents patients who reached radiological CR in cycles 5 or 6 (excluding those who had achieved either 2-cycle CR or 4-cycle CR). The abovementioned statistical analyses were carried out via R software (version 4.3.2; RRID:SCR_001905). Survival analysis was performed with the “survival” package in R. Significance tests were two-tailed, and a P value of less than 0.05 was used to indicate statistical significance.
3. Results
3.1. Patient Characteristics
In this retrospective study, we analysed 1,160 patients with breast cancer who received complete NAT followed by surgical treatment between January 1, 2016, and December 31, 2023, at 3 participating institutions. Among them, 492 (42%) had HR+/HER2- breast cancer, 228 (20%) had HR+/HER2+ breast cancer, 218 (19%) had HR-/HER2+ breast cancer, and 222 (19%) had HR-/HER2- breast cancer. The median follow-up duration for HR+/HER2- patients was 62.3 months (interquartile range [IQR]: 42.8-80.7 months); for HR+/HER2+ patients, it was 53.8 months (IQR: 37.8-79.8 months); for HR-/HER2+ patients, it was 61.4 months (IQR: 43.8-82.1 months); and for HR-/HER2- patients, it was 48.7 months (IQR: 38.1-69.9 months). The baseline patient and tumor characteristics of each subgroup are presented in (Table 1).
Table.
1: Baseline characteristics.
|
Characteristic |
treatment |
||||
|
Overall, N = 1,1601 |
nCR, N = 5021 |
2-cycle CR, N = 2511 |
4-cycle CR, N = 2721 |
6-cycle CR, N = 1351 |
|
|
Age |
51 (43, 58) |
51 (43, 58) |
51 (42, 59) |
52 (44, 57) |
52 (45, 57) |
|
pCR |
|
|
|
|
|
|
npCR |
827 (71.3%) |
475 (94.6%) |
133 (53.0%) |
155 (57.0%) |
64 (47.4%) |
|
pCR |
333 (28.7%) |
27 (5.4%) |
118 (47.0%) |
117 (43.0%) |
71 (52.6%) |
|
T |
|
|
|
|
|
|
1 |
134 (11.6%) |
39 (7.8%) |
41 (16.3%) |
28 (10.3%) |
26 (19.3%) |
|
2 |
766 (66.0%) |
344 (68.5%) |
159 (63.3%) |
179 (65.8%) |
84 (62.2%) |
|
3 |
227 (19.6%) |
108 (21.5%) |
42 (16.7%) |
56 (20.6%) |
21 (15.6%) |
|
4 |
33 (2.8%) |
11 (2.2%) |
9 (3.6%) |
9 (3.3%) |
4 (3.0%) |
|
N |
|
|
|
|
|
|
negative |
271 (23.4%) |
132 (26.3%) |
46 (18.3%) |
59 (21.7%) |
34 (25.2%) |
|
positive |
889 (76.6%) |
370 (73.7%) |
205 (81.7%) |
213 (78.3%) |
101 (74.8%) |
|
Clinical stage |
|
|
|
|
|
|
II |
906 (78.1%) |
380 (75.7%) |
203 (80.9%) |
206 (75.8%) |
117 (86.7%) |
|
III |
254 (21.9%) |
122 (24.3%) |
48 (19.1%) |
66 (24.3%) |
18 (13.3%) |
|
Tumor grade |
|
|
|
|
|
|
1 |
2 (0.2%) |
1 (0.2%) |
0 (0.0%) |
1 (0.4%) |
0 (0.0%) |
|
2 |
908 (78.3%) |
413 (82.3%) |
184 (73.3%) |
209 (76.8%) |
102 (75.6%) |
|
3 |
250 (21.6%) |
88 (17.5%) |
67 (26.7%) |
62 (22.8%) |
33 (24.4%) |
|
SUBTYPE |
|
|
|
|
|
|
HR+/HER2- |
492 (42.4%) |
311 (62.0%) |
70 (27.9%) |
82 (30.1%) |
29 (21.5%) |
|
HR+/HER2+ |
228 (19.7%) |
79 (15.7%) |
54 (21.5%) |
57 (21.0%) |
38 (28.1%) |
|
HR-/HER2+ |
218 (18.8%) |
40 (8.0%) |
67 (26.7%) |
73 (26.8%) |
38 (28.1%) |
|
HR-/HER2- |
222 (19.1%) |
72 (14.3%) |
60 (23.9%) |
60 (22.1%) |
30 (22.2%) |
|
Menopausal status |
|
|
|
|
|
|
Premenopausal
or perimenopausal |
757 (65.3%) |
329 (65.5%) |
151 (60.2%) |
183 (67.3%) |
94 (69.6%) |
|
Postmenopausal |
403 (34.7%) |
173 (34.5%) |
100 (39.8%) |
89 (32.7%) |
41 (30.4%) |
1Median (IQR); n (%).
Among the 492 patients with HR+/HER2- tumors, 65 patients (36%, 28.9-42.9) achieved a pCR. Among the 228 patients with HR+/HER2+ tumors, 63 patients (42.3%, 34.4-50.2) achieved a pCR. Among the 218 patients with HR-/HER2+ tumors, 102 patients (57.3%, 50.0-64.6) achieved a pCR. Among the 222 patients with HR-/HER2- tumors, 76 patients (50.7%, 42.7-58.7) achieved a pCR (Figure 2). The pCR rate was higher in HR-/HER2+ patients than in patients with other subtypes.
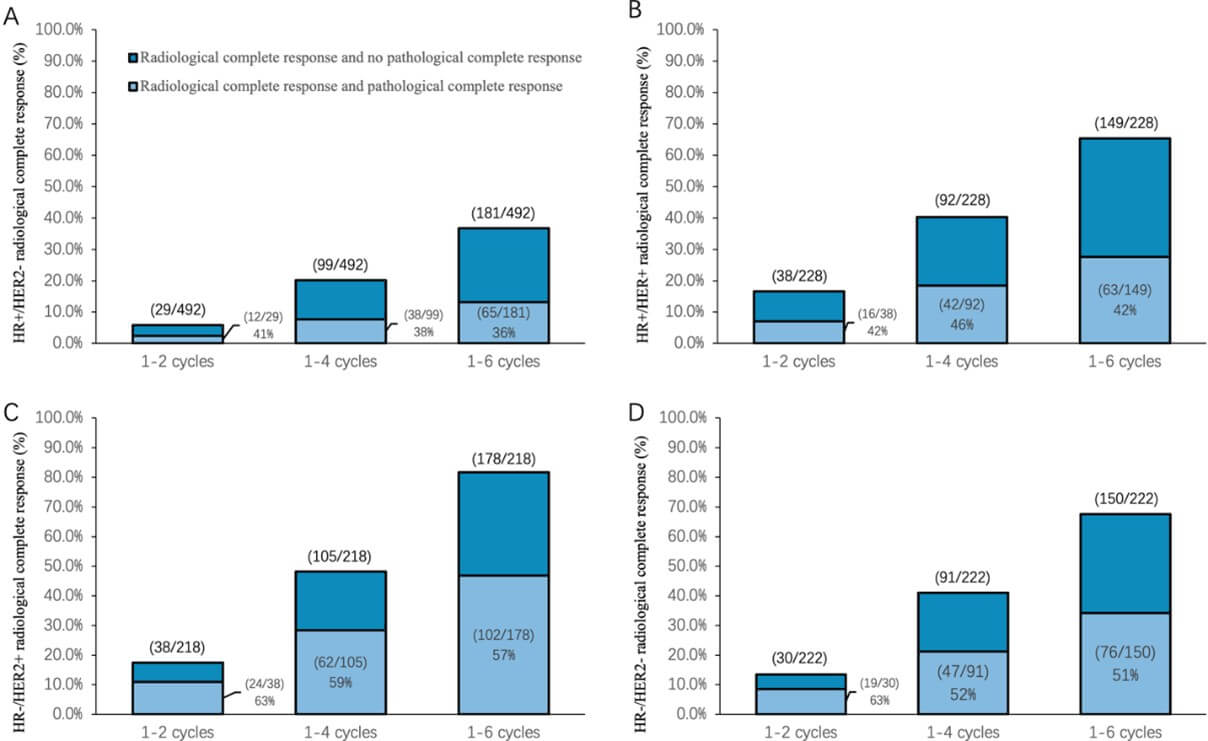
3.2. Primary Outcome: Radiological Response and Survival by Molecular Subtype
To assess the prognostic stratification potential of DCE-MRI based on radiological CR timing during NAT, we conducted comprehensive survival analyses across four biologically distinct breast cancer subtypes. Our investigation specifically evaluated whether the number of treatment cycles required to achieve radiological CR could identify patient subgroups with differential survival outcomes. Furthermore, to establish the clinical utility of DCE-MRI in treatment response monitoring, we performed parallel analyses of both long-term survival outcomes and pathological response rates.
3.3. HR+/HER2- Breast Cancer
The analysis revealed that patients achieving radiological CR at 4 cycles had significantly superior DFS compared to the 6 cycles CR group (P = 0.024; Figure 3A), while no difference was detected between 2 cycles and 6 cycles responders (P > 0.05). When evaluating radiological CR versus nCR patients, those attaining radiological CR within the first four cycles showed improved 8-year DFS (P = 0.024; Figure 3C). Pathologically, radiological CR patients exhibited substantially higher pCR rates (41.38% vs 13.39%; P = 0.0004).
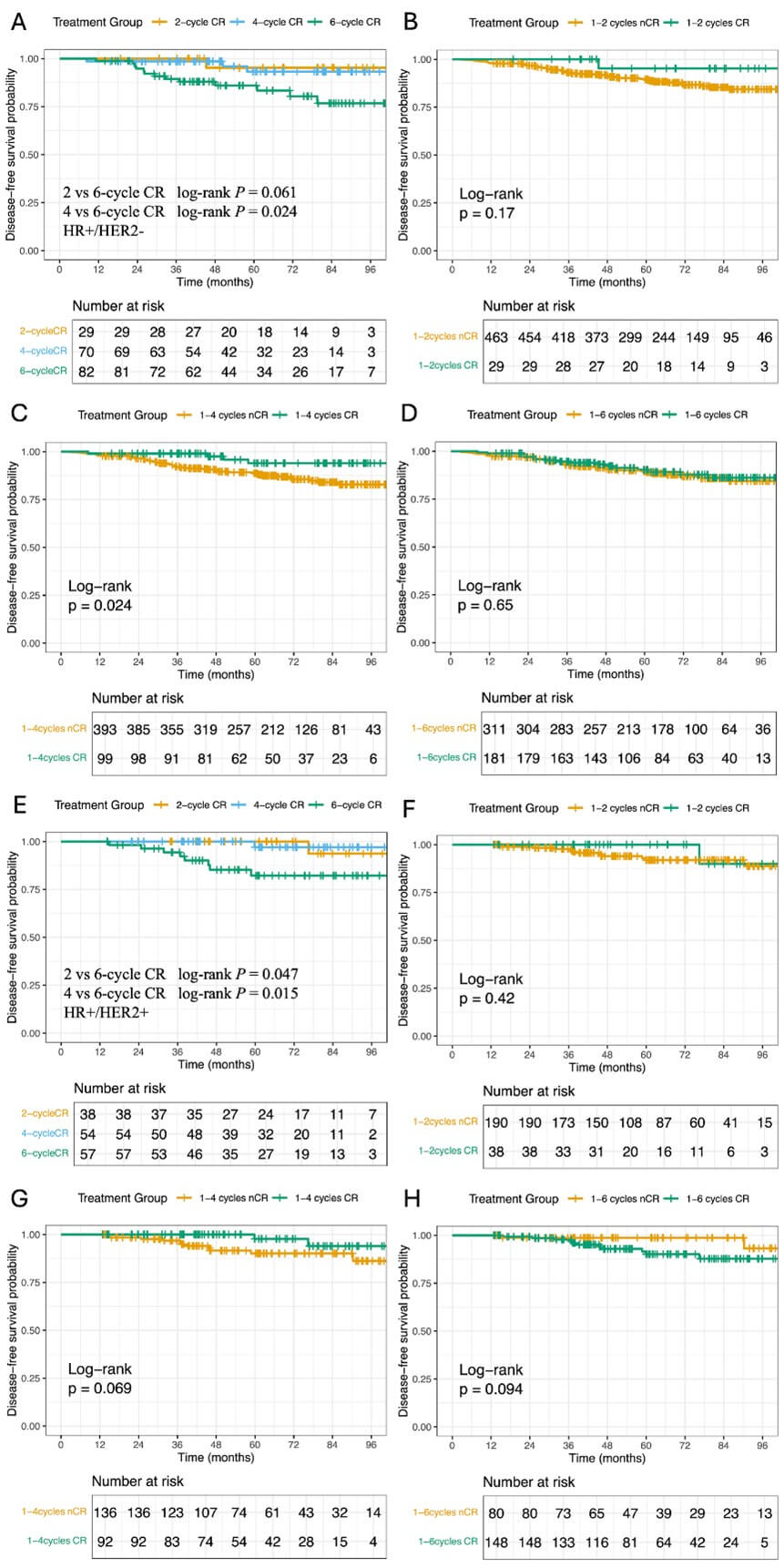
3.4. HR+/HER2+ Breast Cancer
DFS varied significantly across radiological CR timing groups, with both 2 and 4 cycles CR patient demonstrating better outcomes than 6 cycles responders (2 vs. 6 cycles: <>P = 0.047; 4 vs. 6 cycles: P = 0.015; Figure 3E). The 8-year DFS rates were 90.0% (95% CI: 73.2-100) and 96.4% (95% CI: 89.8-100) for early CR groups versus 78.5% (95% CI: 66.5-92.5) for the 6-cycle group. However, radiological CR status did not significantly correlate with DFS differences at any timepoint when compared to nCR patients, consistent with their modest pCR rate disparity (42.11% vs 29.47%; P = 0.1311).
3.5. HR-/HER2+ Breast Cancer
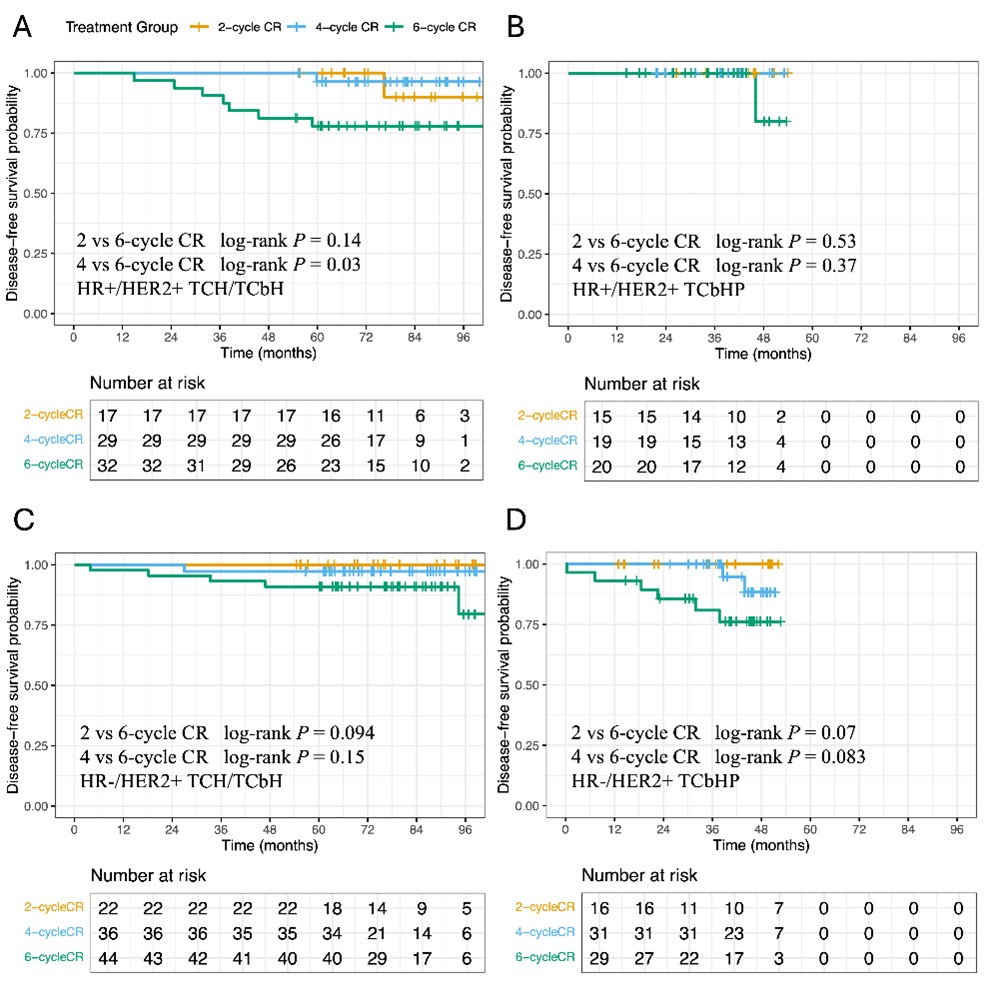
Earlier radiological CR (2 or 4 cycles) was associated with significantly greater DFS (2 vs. 6 cycles: P = 0.013; 4 vs. 6 cycles: P = 0.037; Figure 4A), with 8-year DFS rates of 100% and 94.7% (95% CI: 89.1-100) compared to 74.5% (95% CI: 56.3-98.6) in the 6-cycle group. Radiological CR within four cycles correlated with both DFS improvement (P = 0.0096; Figure 4C) and a trend toward higher pCR rates (63.16% vs 45.00%; P = 0.0498). The prognostic comparison of 2-, 4-, and 6-cycle CR among HER2+ patients receiving different treatment regimens is presented in Supplementary Figure 1 (Figure S1).
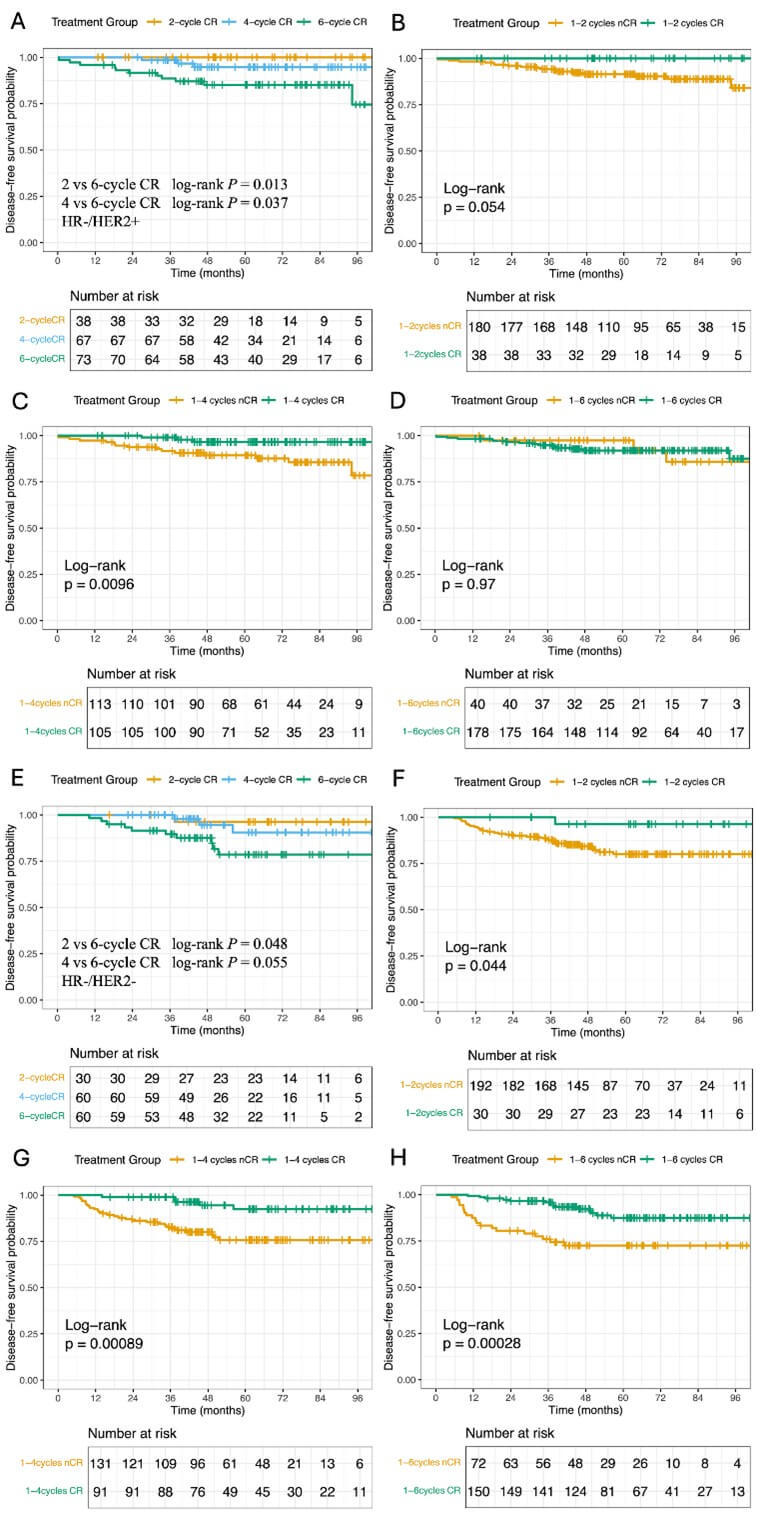
3.6. HR-/HER2- (Triple-Negative) Breast Cancer
Only the difference between 2 cycles and 6 cycles CR groups reached statistical significance in DFS (P = 0.048; Figure 4E). However, radiological CR at all evaluated timepoints (2, 4, or 6 cycles) showed robust survival benefits (1-2 cycles: P = 0.044; Figure 4F; 1-4 cycles: P = 0.00089; Figure 4G; 1-6 cycles: P = 0.00028; Figure 4H). Pathologically, radiological CR patients had nearly doubled pCR rates (63.33% vs 33.85%; P = 0.0039).
These findings collectively suggest that early radiological CR assessment, particularly within the first four treatment cycles, may serve as a valuable predictor for both pCR and long-term survival outcomes, with predictive power varying significantly across molecular subtypes. The strongest associations were observed in HR-/HER2- and HR+/HER2- subtypes, highlighting the importance of tumor biology in interpreting imaging-based response assessments.
4. Discussion
On the basis of the TRAIN-3 study design, this multicenter real-world investigation systematically evaluated the prognostic value of radiological CR after 2, 4, and 6 cycles of NAT, as well as its association with pCR. Unlike the static evaluation methods used in most clinical trials [12], this study innovatively adopted multitime-point dynamic radiological evaluation, which is more in line with actual decision-making needs in clinical practice. In addition, the inclusion of real-world data in China makes the research results more generalizable. The research findings not only verified the important discoveries of previous clinical trials but also provided new clinical evidence for the efficacy evaluation of neoadjuvant treatment for breast cancer.
Our results extend the TRAIN-3 trial’s findings in three key aspects: i) By employing DCE-MRI rather than conventional MRI, we achieved higher sensitivity for early vascular changes. ii) The inclusion of all major subtypes (not limited to HER2+) supports broader applicability. iii) Longitudinal assessment at 2-cycle intervals provides actionable timepoints for therapy modulation.
Previous data have shown that DCE-MRI can accurately assess the size of residual tumors after NAT. Both quantitative and semiquantitative parameters can predict pCR in patients with breast cancer during NAT. However, for breast cancers with different molecular subtypes, the predictive efficiency of these parameters varies [13]. Some studies have indicated that in the HR-/HER2- subgroup, MRI has the highest predictive accuracy for pCR, whereas in the HR-/HER2+ subgroup [14], the false-negative rate is the highest [15]. This study revealed that, for patients with HR+/HER2+ breast cancer, there is a certain degree of consistency between radiological CR and pCR (57.63%). Research has indicated that MRI has the ability to predict pCR [16]. However, that study merely analysed the relationship between MR images obtained before neoadjuvant treatment and pCR. In contrast, this study delved deeper into the occurrence of radiological CR during different cycles of neoadjuvant treatment and their associations with prognosis. In the HR-/HER2+ subgroup, early radiological CR correlated with superior 8-year DFS (94.7% vs 75.6% in late CR). Notably, patients achieving CR within 2 cycles had 100% 8-year DFS- a 25.5% absolute increase over 6-cycle CR. This finding provides a critical reference for the treatment decision-making of HR-/HER2+ patients, suggesting that early radiological evaluation may help identify potential populations that can benefit from reducing the number of chemotherapy cycles. This study provides an earlier indicator of efficacy prediction for use in clinical practice through dynamic radiological evaluation. In patients with triple-negative breast cancer, this study revealed that the prognostic value of radiological CR was relatively limited (34%), which was different from previous research results and may be attributable to the high heterogeneity of TNBC [17, 18]. Although the concordance between radiological CR and pCR varies across different molecular subtypes, early radiological CR still has certain prognostic value.
Currently, international cutting-edge research focuses on strategies to safely avoid surgery and reduce the use of adjuvant therapies. The PHERGain study is a prime example, showing that patients responsive to trastuzumab and pertuzumab, when treated with these agents alone (omitting chemotherapy), achieved an impressive 98.8% 3-year invasive disease-free survival (iDFS), marking a breakthrough in neoadjuvant de-escalation [19]. Similarly, the FASCINATE-N trial demonstrated that SHR-A1811 had comparable efficacy to the standard TCbHP regimen for the neoadjuvant treatment of HER2+ breast cancer, with better safety and lower discontinuation and dose adjustment rates [20]. These findings echo global efforts to optimize neoadjuvant de-escalation for HER2+ breast cancer [20, 21]. Our study further supports the potential for treatment deintensification in this patient group. Moreover, our data reveal a similar trend in HR-/HER2- breast cancer, collectively contributing to improved treatment strategies and patient outcomes.
Several limitations warrant consideration. First, the retrospective design may introduce selection bias, though we mitigated this through multivariable adjustment. Second, at the technical level, this study used DCE-MRI, and at least three professional radiologists were involved in determining whether patients achieved radiological CR. In clinical practice, achieving joint interpretation of results by multiple radiologists is difficult. Finally, while we excluded patients with incomplete NAT, real-world treatment adherence may further influence imaging-survival correlations—a factor meriting prospective validation.
Overall, this study provides reliable support for the early treatment response evaluation of breast cancer patients, confirms the necessity of dynamic radiological monitoring in real-world clinical scenarios, and lays a theoretical foundation for the individualized adjustment of treatment results in regimens. For HR-/HER2- disease, combining functional imaging with circulating tumor DNA or immune markers may enhance prediction accuracy. Future trials should test whether early CR can safely guide NAT de-escalation, potentially reducing overtreatment without compromising survival outcomes.
Author Contributions
YQZ, FJ, and AZ developed the study concept and established the research methodology. ZXD, PS, and FY were responsible for clinical data acquisition, management, statistical evaluation, and results verification. YZ and MYP handled patient enrollment and maintained ethical standards. MLCDLZ performed in-depth data analysis and outcome evaluation. The initial manuscript was prepared by ZXD and MLC, with contributions from all co-authors. All authors critically reviewed and endorsed the final manuscript.
Conflicts of Interest
None.
Ethics Statement
This study, involving retrospective analysis of anonymized patient data, was conducted in accordance with ethical guidelines. Individual informed consent was waived because the research posed no risk to participants and used only de-identified records.
Funding
This study was supported by the National Natural Science Foundation of China [82203873 (AZ)], the Science and Technology Joint Plan of Liaoning Province - Surface Funding Project [2023012141-JH3/4600(AZ)], and the Science and Technology Joint Plan of Liaoning Province - Technological Breakthrough Project [2024JH2/10260031(FJ)].
Data Availability Statement
The datasets produced and analysed during the current study are available from the corresponding author upon reasonable request.
Acknowledgements
We sincerely express our gratitude to the participating institution, the Affiliated Zhongshan Hospital of Dalian University (Xiaolan Wang). Since the initiation of this study in 2016, they have been actively involved in this project.
Abbreviations
CR: Complete Response
DFS: Disease-Free Survival
nCR: Noncomplete Response
pCR: Pathological Complete Response
NAT: Neoadjuvant Therapy
DCE-MRI: Dynamic Contrast-Enhanced Magnetic Resonance Imaging
TNBC: Triple-Negative Breast Cancer
IHC: Immunohistochemistry
FISH: Fluorescence In Situ Hybridization
CT: Computed Tomography
REFERENCES
[1] Freddie
Bray, Mathieu Laversanne, Hyuna Sung, et al. “Global cancer statistics 2022:
GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185
countries.” CA Cancer J Clin, vol.
74, no. 3, pp. 229-263, 2024. View at: Publisher Site | PubMed
[2] Javier
David Benitez Fuentes, Eileen Morgan, Alicia de Luna Aguilar, et al. “Global
Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and
Meta-Analysis.” JAMA Oncol, vol. 10,
no 1, pp. 71-78, 2024. View at: Publisher Site | PubMed
[3] Patricia
Cortazar, Lijun Zhang, Michael Untch, et al. “Pathological complete response
and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis.” Lancet, vol. 384, no. 9938, pp. 164-172,
2014. View at: Publisher Site | PubMed
[4] Peter
McAnena, Brian M Moloney, Robert Browne, et al. “A radiomic model to classify
response to neoadjuvant chemotherapy in breast cancer.” BMC Med Imaging, vol. 22, no. 1, pp. 225, 2022. View at: Publisher Site | PubMed
[5] Domenico
Albano, Federico Bruno, Andrea Agostini, et al. “Dynamic contrast-enhanced
(DCE) imaging: state of the art and applications in whole-body imaging.” Jpn J Radiol, vol. 40, no. 4, pp. 341-366, 2022. View at: Publisher Site | PubMed
[6] Yunju
Kim, Sung Hun Kim, Byung Joo Song, et al. “Early Prediction of Response to
Neoadjuvant Chemotherapy Using Dynamic Contrast-Enhanced MRI and Ultrasound in
Breast Cancer.” Korean J Radiol, vol.
19, no. 4, pp. 682-691, 2018. View at: Publisher Site | PubMed
[7] Xia
Li, Lori R Arlinghaus, Gregory D Ayers, et al. “DCE-MRI analysis methods for
predicting the response of breast cancer to neoadjuvant chemotherapy: pilot
study findings.” Magn Reson Med, vol.
71, no. 4, pp. 1592-1602, 2014. View at: Publisher Site | PubMed
[8] Elizabeth
A M O'Flynn, David Collins, James D'Arcy, et al. “Multi-parametric MRI in the
early prediction of response to neo-adjuvant chemotherapy in breast cancer:
Value of non-modelled parameters.” Eur J
Radiol, vol. 85, no. 4, pp. 837-842, 2016. View at: Publisher Site | PubMed
[9] Takayo
Fukuda, Rie Horii, Naoya Gomi, et al. “Accuracy of magnetic resonance imaging
for predicting pathological complete response of breast cancer after
neoadjuvant chemotherapy: association with breast cancer subtype.” Springerplus, vol. 5, pp. 152, 2016.
View at: Publisher Site | PubMed
[10]
Henry M Kuerer, Vicente Valero,
Benjamin D Smith, et al. “Selective Elimination of Breast Surgery for Invasive
Breast Cancer: A Nonrandomized Clinical Trial.” JAMA Oncol, vol. 11, no. 5, pp. 529-534, 2025. View at: Publisher Site | PubMed
[11]
van der Voort A, Louis FM, van
Ramshorst MS, et al. “MRI-guided optimisation of neoadjuvant chemotherapy
duration in stage II-III HER2-positive breast cancer (TRAIN-3): a multicentre,
single-arm, phase 2 study.” Lancet Oncol,
vol. 25, no. 5, pp. 603-613, 2025. View at: Publisher Site | PubMed
[12]
Kazuhiro Kitajima, Yasuo Miyoshi,
Toshiko Yamano, et al. “Assessment of tumor response to neoadjuvant
chemotherapy in patients with breast cancer using MRI and FDG-PET/CT-RECIST 1.1
vs. PERCIST 1.0.” Nagoya J Med Sci,
vol. 80, no. 2, pp. 183-197, 2018. View at: Publisher Site | PubMed
[13]
Jieun Kim, Boo-Kyung Han, Eun Young
Ko, et al. “Prediction of pathologic complete response on MRI in patients with
breast cancer receiving neoadjuvant chemotherapy according to molecular
subtypes.” Eur Radiol, vol. 32, no.
6, pp. 4056-4066, 2022. 20220106. View at: Publisher Site | PubMed
[14]
L M Janssen, B M den Dekker, K G A
Gilhuijs, et al. “MRI to assess response after neoadjuvant chemotherapy in
breast cancer subtypes: a systematic review and meta-analysis.” NPJ Breast Cancer, vol. 8, no. 1, pp.
107, 2022. View at: Publisher Site | PubMed
[15]
Monika Graeser, Simone Schrading,
Oleg Gluz, et al. “Early response by MR imaging and ultrasound as predictor of
pathologic complete response to 12-week neoadjuvant therapy for different early
breast cancer subtypes: Combined analysis from the WSG ADAPT subtrials.” Int J Cancer, vol. 148, no. 10, pp.
2614-2627, 2021. View at: Publisher Site | PubMed
[16]
Zhenyu Liu, Zhuolin Li, Jinrong Qu,
et al. “Radiomics of Multiparametric MRI for Pretreatment Prediction of
Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A
Multicenter Study.” Clin Cancer Res,
vol. 25, no. 12, pp. 3538-3547, 2019. View at: Publisher Site | PubMed
[17]
Lin Jiang, Chao You, Yi Xiao, et al.
“Radiogenomic analysis reveals tumor heterogeneity of triple-negative breast
cancer.” Cell Rep Med, vol. 3, no. 7,
pp. 100694, 2022. View at: Publisher Site | PubMed
[18]
Eva Kudelova, Marek Smolar, Veronika
Holubekova, et al. “Genetic Heterogeneity, Tumor Microenvironment and
Immunotherapy in Triple-Negative Breast Cancer.” Int J Mol Sci, vol. 23, no. 23, pp. 20221129, 2022. View at: Publisher
Site | PubMed
[19]
José Manuel Pérez-García, Javier
Cortés, Manuel Ruiz-Borrego, et al. “3-year invasive disease-free survival with
chemotherapy de-escalation using an (18)F-FDG-PET-based, pathological complete
response-adapted strategy in HER2-positive early breast cancer (PHERGain): a
randomised, open-label, phase 2 trial.” Lancet,
vol. 403, no. 10437, pp. 1649-1659, 2024. View at: Publisher Site | PubMed
[20]
Ding Ma, Lei-Jie Dai, Xiang-Rong Wu,
et al. “Spatial determinants of antibody-drug conjugate SHR-A1811 efficacy in
neoadjuvant treatment for HER2-positive breast cancer.” Cancer Cell, vol. 43, no. 6, pp. 1061-1075.e7, 2025. View at: Publisher Site | PubMed
[21] Ulrike Nitz, Oleg Gluz, Monika Graeser, et al. “De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR-): survival outcomes from a multicentre, open-label, randomised, phase 2 trial.” Lancet Oncol, vol. 23, no. 5, pp. 625-635, 2022. View at: Publisher Site | PubMed
