Received: Sat 02, Sep 2023
Accepted: Wed 27, Sep 2023
Abstract
Objective: Acute aortic dissection (AAD) has a high rate of mortality and postoperative complications, but fewer indicators can predict postoperative cardiac injury complications. This study aimed to investigate whether α-HBDH is superior to LDH as a predictor of in-hospital mortality and postoperative cardiac injury in patients with AAD. Methods: In this retrospective study, a total of 369 patients from 2015 to 2021 were enrolled and divided into three groups (T1: low, T2: medium and T3: high) based on the tertiles of α-HBDH levels on admission. The relationship between α-HBDH and AAD was determined by correlation analysis and logistic regression model, with the consolidation using Kaplan-Meier and restricted cubic spline (RCS) analysis for predicting the in-hospital death and cardiac injury complications. Last, the relationship between α-HBDH levels and in-hospital mortality and cardiac injury was further verified through subgroup analysis. Results: In the logistic regression model, α-HBDH in the high-level group was 9.869 times that in the low-level group [OR (95CI): 9.869 (2.148-45.349), P=0.003], while this feature was not observed in LDH [OR (95CI): 1.437 (0.342-6.034), P=0.621]. Kaplan-Meier analysis showed that increased α-HBDH levels within 30 days were associated with poor survival within 30 days in AAD patients (log rank test, P<0.01), especially in acute stanford A dissection. RCS showed that 204 U/L was the optimal cut-off value of α-HBDH for in-hospital mortality and postoperative cardiac injury, which facilitated clinical stratification of patients with AAD. Subgroup analysis confirmed a stable correlation between α-HBDH level and hospital mortality and cardiac injury (P>0.05). Conclusion: α-HBDH would be better than LDH in predicting the in-hospital death and postoperative cardiac injury, guiding admission stratification of patients with AAD.
Keywords
Acute aortic dissection, α-HBDH, LDH, cardiac injury
1. Introduction
Acute aortic dissection (AAD) is a severe aortic disease with a high morbidity and mortality [1-4]. Surgical correction of AAD should be performed as soon as possible; otherwise, patients may die from aortic rupture, pericardial tamponade, aortic insufficiency, and heart failure. According to the early reports of Stanford A AAD (SAAAD), which is a type of AAD that begins from the ascending aorta, the perioperative mortality rate is 30%-60% [5]. With the improvement in treatments and postoperative management, the perioperative mortality rate has decreased to 13%-25% [2], whereas the risk of early postoperative complications affecting the nervous, respiratory, digestive, and circulatory systems was still 58.3% [6]. Furthermore, cardiac injury following AAD surgery is associated with a high mortality and poor prognosis.
α-hydroxybutyrate dehydrogenase (α-HBDH), which is found in the myocardium, kidneys, and red blood cells, reflects the activity of H-type lactate dehydrogenase (LDH1 and LDH2). Levels of α-HBDH are highest in the myocardium as an auxiliary marker of cardiac injury [7-9]. AAD initially reduces coronary blood supply, leading to cardiac ischemia and apoptosis if early intervention is not initiated. There have been reports on the relationship between LDH levels and in-hospital mortality from AAD [10]; however, it remains unclear whether α-HBDH is associated with the initiation and progression of AAD as well as cardiac injury.
Recent evidence indicates that the preoperative serum α-HBDH level is associated with in-hospital mortality, cardiac injury, and postoperative intensive care admission (unplanned intensive care unit admissions) after non-cardiac surgery [11]. Meanwhile, the predictive value of α-HBDH for cardiac infarction has been reported, which contributes to the hypothesis that it may also represent a poor prognosis following cardiac ischemia in patients with AAD [12]. The aim of this study was to explore the prognostic ability of α-HBDH on postoperative in-hospital mortality and cardiac complications of patients with AADs.
2. Materials and Methods
2.1. Patients
We retrospectively reviewed the data of 369 consecutive patients with AAD seen at the cardiac surgery or emergency department, Fourth Hospital of Hebei Medical University between January 2015 and June 2021. Diagnosis of AAD in all patients (aged 18 years or older) was confirmed by computed tomographic angiography, with duration of onset ≤14 days as definitive diagnosis. All patients with complete data, including α-HBDH and brain natriuretic peptide levels and cardiac troponin Ⅰ results, that were available on hospital admission as well as those who developed symptoms within 72 hours were included in the study. The exclusion criteria were as follows: patients with incomplete data, Marfan’s syndrome, pregnancy, traumatic dissection, and chronic aortic dissection. Using the tertiles of the α-HBDH level on admission, patients were divided into three groups: T1, ≤178 U/L (n = 126); T2, 179-259 U/L (n = 122); and T3, ≥ 260 U/L (n = 121).
Collection and analysis of demographic and clinical data were approved by the ethics committee of the Fourth Hospital of Hebei Medical University (2021k7359). As patient data were anonymized, the ethics committee of the Fourth Hospital of Hebei Medical University waived the written informed consent. All procedures followed were in accordance with the revised Declaration of Helsinki.
2.2. Clinical Data Collection
Clinical variables were collected from the patients’ medical records and included gender, age, past medical history (hypertension, diabetes, coronary heart disease, surgical history), cigarette smoking, alcohol intake, systolic blood pressure (SBP), diastolic blood pressure, type of AAD (stanford A or B), length of hospital stay, and in-hospital mortality. Cigarette smoking was defined smoking every day for more than 6 months. Drinking was defined as alcohol consumption ≥25 g per day or ≥100 g per week for more than 1 year.
2.3. Laboratory Test Indicators
Peripheral blood sample collection was performed prior to any surgical/endovascular treatment. Levels of α-HBDH (reference range, 72-182 U/L), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), urea (BUN), glucose, platelet count, hemoglobin, red blood cell count, white blood cell count, neutrophil count, lymphocyte count, mononuclear cell count, LDH, and serum K+, Na+, Cl−, and Ca2+ were measured.
2.4. AAD Therapy
Patients with AAD complicated with hypertension or other chronic diseases were intravenously administered hypotensive drugs or nitroglycerin preoperatively to maintain the SBP at 100-120 mmHg. Patients with type A AAD underwent surgical repair (aortic root treatment, total arch replacement and distal elephant trunk surgery or hybrid surgery) under extracorporeal circulation, while patients with type B AAD underwent interventional therapy according to its location and severity (heart and vascular lesions). Complicated type B AAD was treated with thoracic endovascular aortic repair. If cases of type B AAD with very low risk of retrograde tear and vascular risk were encountered, they were repaired with cardiopulmonary bypass.
2.5. Definition of Renal Insufficiency, Hepatic Insufficiency, and Cardiac Injury
Renal insufficiency was defined as a decrease of ≥30% in the glomerular filtration rate or effective renal plasma flow compared with baseline values [13-15]. Acute kidney injury was defined as an increase in serum creatinine levels >0.3 mg/dL or 1.5 times above baseline values within 7 days. Hepatic insufficiency was diagnosed when ALT or AST levels were >80 U/L, or there were signs of liver failure. Cardiac injury was diagnosed when the brain natriuretic peptide level was >500 pg/mg, cardiac troponin level was >1.5 µg/L, or there were postoperative complications such as cardiac infarction, pericardial tamponade, pericardial effusion, and heart failure.
2.6. Study Outcomes and Follow-up
In-hospital mortality and length of hospitalization were obtained from medical records. All-cause mortality during hospitalization was defined as the primary endpoint, and the secondary endpoint was the incidence of cardiac injury or other complications.
2.7. Statistical Analysis
Data with normal distribution were expressed as mean ± standard deviation, and one-way analysis of variance was used to compare more than two groups. Non-normally distributed variables were expressed as median (P25, P75). The Kruskal-Wallis H test was used for multi-group comparison in terms of continuous variables. Categorical variables were shown as frequency (%) and compared by the Chi-square test and Fisher’s exact test. Multivariate logistic regression analysis was used to determine factors associated with the postoperative prognosis in terms of the odds ratio and 95% confidence interval. In multivariate regression analysis, potential confounders were adjusted (P < 0.05). Kaplan-Meier curves were created to determine the cumulative survival of each group through the log rank test for comparison. RCS was used to explore the relationship between α-HBDH and in-hospital mortality and cardiac injury and to determine the optimal value for stratification. Subgroup analyses for age, gender, hypertension, AST and Cr levels, smoking and drinking, type of AAD, and LDH level at baseline were performed with tests for interaction. SPSS 25.0 and R version 4.2.1 were used for all statistical analyses. P < 0.05 was considered statistically significant. Patient characteristics, principal results, and implications of this study are shown in (Figure 1).
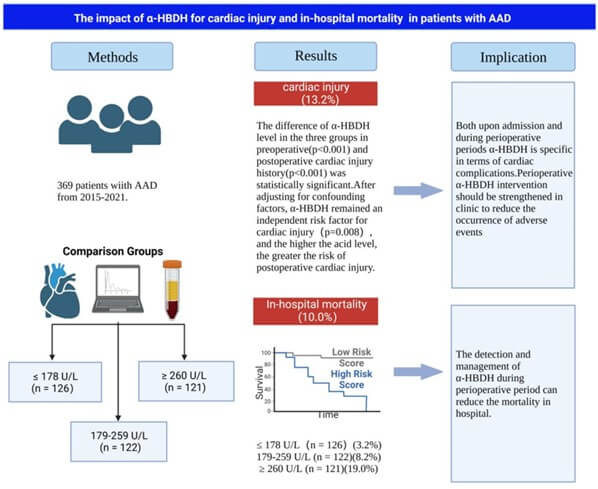
3. Results
3.1. Characteristics of Patients with AAD
There were 369 patients with AAD with a mean age of 53.2 (44.5, 63.0) years; 273 (74%) were men. The clinical features at baseline are displayed in (Table 1). The median duration of hospitalization was 12 days. Characteristics of patients included hypertension (73.2%), diabetes (1.9%), coronary artery disease (4.1%), drinking (58.5%), and smoking (53.4%). Compared to baseline values, changes in ALT, AST, creatinine, BUN, blood glucose, and LDH levels during hospitalization were all significant (all P<0.05).
TABLE
1: Baseline characteristics of patients with AAD.
|
Characteristic |
Total (n=369) |
Tertile I (≤178 U/L) (n=126) |
Tertile II (179-259 U/L) (n=122) |
Tertile III (≥260 U/L) (n=121) |
P-value |
|
Demographics |
|
|
|
|
|
|
Age(y) |
53.0 (44.5, 63.0) |
54.0 (47.8, 64.0) |
53.0 (44.5, 64.0) |
51.0 (41.0, 62.0) |
0.107 |
|
Gender(male) |
273.0 (74.0) |
93.0 (73.8) |
94.0 (77.0) |
86.0 (71.1) |
0.569 |
|
Comorbidities |
|
|
|
|
|
|
Hypertension |
270.0 (73.2) |
96.0 (76.2) |
82.0 (67.2) |
92.0 (76.0) |
0.193 |
|
Diabetes |
7.0 (1.9) |
3.0 (2.4) |
2.0 (1.6) |
2.0 (1.6) |
0.887 |
|
CHD |
15.0 (4.1) |
5.0 (4.1) |
5.0 (4.1) |
5.0 (4.1) |
0.998 |
|
Smoking |
197.0 (53.4) |
68.0 (54.0) |
73.0 (59.8) |
56.0 (46.3) |
0.105 |
|
Drinking |
76.0 (58.5) |
74.0 (58.7) |
66.0 (54.1) |
66.0 (54.1) |
0.720 |
|
History of cardiac injurya |
30.0(8.1) |
3.0(2.4)b |
5.0(4.1)c |
22.0(18.2) |
<0.001 |
|
History of Surgery |
321.0 (87.0) |
113.0 (89.7) |
106.0 (86.9) |
102.0 (84.3) |
0.453 |
|
Type of AAD |
216.0 (58.5) |
67.0 (53.2) |
71.0 (58.2) |
78.0 (64.5) |
0.198 |
|
|
|
|
|
|
|
|
SBP |
136.0 ± 28.5 |
140.8 ± 25.5 |
138.7 ± 30.0 |
139.3 ± 29.1 |
0.833 |
|
DBP |
81.0 ± 19.0 |
81.2 ± 17.6 |
80.5 ± 20.6 |
81.3 ± 18.9 |
0.937 |
|
ALT |
22.2 (15.6, 31.5) |
18.9 (13.9, 29.4) |
21.2 (15.5, 31.2)c |
25.2 (17.9, 34.9) |
0.002 |
|
AST |
29.9 (19.0, 49.7) |
21.9 (17.8, 35.2)b |
28.7 (20.4, 47.0)c |
43.7 (26.1, 72.4) |
<0.001 |
|
Creatinine |
79.0 (62.0, 108.0) |
70.0 (57.0, 92.0) |
77.0 (62.0, 99.0)c |
96.0 (73.0, 130.0) |
<0.001 |
|
BUN |
6.1 (4.7, 8.1) |
5.6 (4.4, 7.5) |
6.0 (4.9, 7.9)c |
6.8 (5.1, 9.1) |
0.002 |
|
Glucose |
7.1 (6.0, 8.8) |
6.8 (5.8, 8.0) |
7.2 (6.1, 8.5)c |
7.3 (6.2, 9.4) |
0.044 |
|
Hematologic signatures |
|
|
|
|
|
|
Platelet |
171.5 (134.0, 211.0) |
177.5 (137.8, 216.5) |
165.5 (133.0, 203.8) |
171.5 (133.3, 213.3) |
0.415 |
|
HB |
128.3 ± 17.0 |
128.6 ± 15.2 |
128.0 ± 18.0 |
128.5 ± 18.0 |
0.960 |
|
RBC |
4.1 ± 0.7 |
4.1 ± 0.5 |
4.1 ± 0.6 |
4.2 ± 0.7 |
0.482 |
|
WBC |
11.5 (9.4, 14.2) |
11.3 (9.4, 13.5) |
11.7 (9.5, 14.5) |
11.6 (9.3, 14.2) |
0.900 |
|
NEUT |
9.8 (7.5, 12.5) |
9.8 (7.2, 11.9) |
9.8 (7.5, 12.9) |
10.0 (7.5, 12.4) |
0.934 |
|
LYM |
0.9 (0.7, 1.3) |
1.0 (0.7, 1.4) |
0.8 (0.7, 1.2) |
0.9 (0.7, 1.4) |
0.051 |
|
LDH |
232.9 (189, 349.3) |
175.8 (156.7, 206.3)b |
233.8 (208.8, 281.7)c |
380.0 (293.5, 472.8) |
<0.001 |
|
Electrolytes signatures |
|
|
|
|
|
|
Serum K+ |
4.0 (3.4, 4.0) |
4.0 (3.4, 4.0) |
3.9 (3.4, 4.0) |
4.0 (3.5, 4.2) |
0.339 |
|
Serum Na+ |
138.0(136.0,140.0) |
138.0(135.7,140.0) |
139.0(136.0,141.0) |
138.0(136.0,141.0) |
0.082 |
|
Serum Cl- |
104.0 (101.0, 107.0) |
138.0 (135.8, 139.3) |
139.0 (136.0, 141.0) |
138.0 (136.0, 141.0) |
0.093 |
|
Serum Ca2+ |
2.2 (2.1, 2.3) |
2.2 (2.1, 2.3) |
2.2 (2.1, 2.3) |
2.2 (2.1, 2.3) |
0.758 |
CHD:
Coronary Heart Disease; SBP: Systolic Blood Pressure; DBP: Diastolic Blood
Pressure; ALT: Alanine Transaminase; AST: Aspartate Transaminase; BUN: Blood
Urea Nitrogen; HB: Hemoglobin; RBC: Red Blood Cell Count; WBC: White Blood Cell
Count; NEUT: Neutrophil Count; LYM: Lymphocyte Count; MONO: Mononuclear Cell
Count; LDH: Lactic Dehydrogenase.
aPatients with any one of preoperative
comorbidities including pericardial effusion, tamponade, cardiac infarction,
cardiac insufficiency, cardiogenic shock, heart failure etc.
pericardial
tamponade, pericardial effusion, and heart failure.
bThe comparison between T1 and T3
group P<0.05.
cThe comparison between T2
and T3 group P<0.05.
3.2. Short-term Outcomes of Patients with AAD
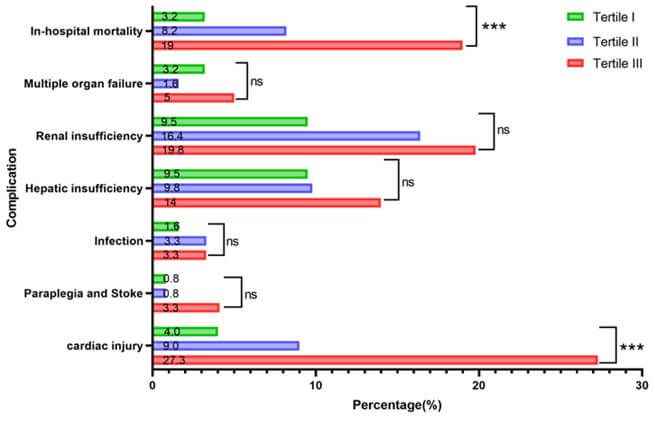
Table 2 shows the short-term postoperative outcomes of patients with AAD. The overall in-hospital mortality was 10%; the mortality rates in the T1, T2, and T3 groups were 3.2%, 8.2%, and 19%, respectively, showing a tendency for mortality with increased α-HBDH levels. There were statistically significant differences in the incidence of cardiac injury among the three groups (4%, 9%, 27.3%, respectively; all P <0.001). However, there were no significant differences in the incidences of other conditions, including multiple organ failure, hepatic insufficiency, infection, paraplegia, and stroke, among all groups (all P>0.05; Figure 2). These results suggest that elevated levels of α-HBDH were significantly associated with cardiac injury in patients with AAD (P<0.05).
TABLE 2: Short-term outcomes of patients with AAD.
|
outcomes |
Total (n=369) |
Tertile I (≤178 U/L) (n=126) |
Tertile II (179-259 U/L) (n=122) |
Tertile III (≥260 U/L) (n=121) |
P-value |
|
In-hospital mortality |
37.0 (10.0) |
4.0 (3.2) |
10.0 (8.2)c |
23.0 (19.0) |
<0.001 |
|
Multiple organ failure |
12.0(3.3) |
4.0(3.2) |
2.0(1.6) |
6.0(5.0) |
0.345 |
|
Renal insufficiency |
56.0(15.2) |
12.0(9.5) |
20.0(16.4) |
24.0(19.8) |
0.07 |
|
Hepatic insufficiency |
41.0(11.1) |
12.0(9.5) |
12(9.8) |
17(14) |
0.454 |
|
Infection |
10.0(2.7) |
2.0(1.6) |
4.0(3.3) |
4.0(3.3) |
0.633 |
|
Paraplegia and Stroke |
7.0(1.2) |
1.0(0.8) |
1.0(0.8) |
5.0(4.1) |
0.089 |
|
Cardiac injurya |
49(13.2) |
5.0(4.0)b |
11.0(9.0)c |
33.0(27.3) |
<0.001 |
|
Hospital stays |
12.0 (6.0, 20.0) |
12.0 (5.0, 19.0) |
12.0 (7.8, 21) |
11.0 (3.0, 20.5) |
0.311 |
aPatients with any one of
postoperative comorbidities including brain natriuretic peptide (BNP) > 500
pg/mg or Cardiac troponin (cTnI) > 1.5µg/L, or postoperative complications
of cardiac injury such as cardiac infarction.
bThe comparison between T1 and T3
group P<0.05.
cThe comparison between T2
and T3 group P<0.05.
TABLE 3: Multivariable
Logistic Model of In-hospital mortality and Cardiac injury with α-HBDH and LDH.
|
|
|
In-hospital mortality |
cardiac injury |
||||||
|
|
|
α-HBDH(U/L) |
Tertile I |
Tertile II |
Tertile III |
α-HBDH(U/L) |
Tertile I |
Tertile II |
Tertile III |
|
|
|
|
OR(95CI) P |
OR(95CI) P |
OR(95CI) P |
|
OR(95CI) P |
OR(95CI) P |
|
|
Model1 |
Crude |
1.007(1.005-1.010)<0.001 |
1.000 |
2.723(0.830-8.930)0.099 |
7.158(2.396-21.388)
<0.002 |
1.005(1.002-1.007)
<0.001 |
1.000 |
2.398(0.808-7.119)
0.115 |
9.075(3.406-24.176)
<0.001 |
|
Model2 |
Adjusted
for demographicsa |
1.008(1.005-1.011)<0.001 |
1.000 |
2.829(0.860-9.309)
0.087 |
7.673(2.544-23.146)
<0.001 |
1.008(1.005-1.010)
<0.001 |
1.000 |
2.413(0.812-7.174)
0.113 |
9.406(3.506-25.235)
<0.001 |
|
Model3 |
Adjusted
for demographics, comorbiditiesb, type of AADc, Admission
indictorsd |
1.007(1.004-1.010)<0.001 |
1.000 |
2.207(0.622-7.820
) 0.220 |
6.534(1.998-21.370)
0.002 |
1.005(1.001-1.009)
0.013 |
1.000 |
2.902(0.702-11.998)
0.141 |
7.366(1.947-27.875)
0.003 |
|
Model4 |
Adjusted
for demographics, comorbidities, type of AAD, admission indictors and lab
teste |
1.008(1.003-1.013)
0.001 |
1.000 |
2.326(0.521-10.381)
0.269 |
4.771(1.043-21.832)
0.044 |
1.006(1.002,1.011)
0.008 |
1.000 |
3.062(0.638-14.696)
0.162 |
9.869(2.148-45.349)
0.003 |
|
|
|
LDH(U/L) |
Tertile I |
Tertile II |
Tertile III |
LDH(U/L) |
Tertile I |
Tertile II |
Tertile III |
|
|
|
OR(95CI) P |
OR(95CI) P |
OR(95CI) P |
OR(95CI) P |
OR(95CI) P |
OR(95CI) P |
||
|
Model1 |
Crude |
1.004(1.002-1.006)
<0.001 |
1.000 |
3.821(1.221-11.962)
0.021 |
5.435(1.791-16.490)
0.003 |
1.000(1.000-1.001)
0.341 |
1.000 |
1.101(0.466-2.599)
0.827 |
2.729(1.282-5.810)
0.009 |
|
Model2 |
Adjusted
for demographics |
1.004(1.002-1.006)
<0.001 |
1.000 |
3.966(1.262-12.467)
0.018 |
5.838(1.910-17.843)
0.002 |
1.077(0.529-2.194)
0.838 |
1.000 |
1.100(0.465-2.599)
0.828 |
2.741(1.283-5.853)
0.009 |
|
Adjusted for demographics, comorbidities,
type of AAD, Admission indictors |
1.004(1.001-1.006)0.002 |
1.000 |
3.807(1.101-13.173)0.035 |
5.842(1.674-20.386)
0.006 |
0.325(1.000-1.001)
0.325 |
1.000 |
0.675(0.185-2.461)
0.552 |
2.721(0.895-8.270)
0.078 |
|
|
Model4 |
Adjusted
for demographics, comorbidities, type of AAD, Admission indictors and lab test |
1.000(0.998,1.003)
0.700 |
1.000 |
3.188(0.724-14.040)
0.125 |
1.605(0.277-9.293)
0.598 |
0.997(0.994-1.001)
0.147 |
1.000 |
0.411(0.094-1.789)
0.236 |
1.437(0.342-6.034)
0.621 |
Values are HR (95% CI) aDemographics
including age and gender. bComorbidities including hypertension,
diabetes, CHD, smoking, drinking, history of cardiac injury, history of
surgery, cType of AAD including stanford A and stanford B. dAdmission
indictors including SBP, DBP, creatinine, BUN, glucose. eLab test
including Platelet, HB, RBC, WBC, NEUT, LYM, Serum K+, Serum Na+,
Serum Cl-, Serum.
3.3. Multivariable Logistic Regression Model of in Hospital Mortality and Cardiac Injury According to α-HBDH and LDH Levels
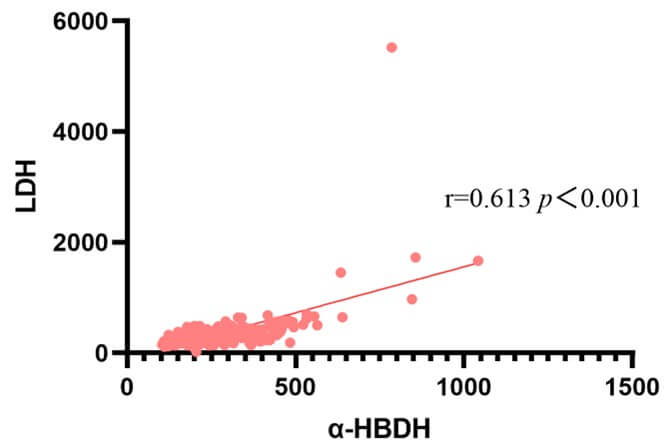
As α-HBDH could better reflect levels of LDH1 and LDH2, we explored the relationship between α-HBDH and LDH (Supplementary Figure 1). Pearson correlation showed that there was a significantly positive correlation between α-HBDH and LDH (r = 0.613, P<0.001; Figure 3).
We constructed four logistic regression models with the main study endpoint (in-hospital mortality). α-HBDH was first set as a continuous variable, and the four models were adjusted respectively. The results showed that α-HBDH was always an independent risk factor for in-hospital mortality. Afterwards, α-HBDH was set as a categorical variable, and patients were divided into three groups (T1, T2, and T3). The results showed that α-HBDH was still an independent risk factor for in-hospital mortality in the T3 group, with the T1 group as reference. Regarding the secondary endpoint (cardiac injury), when α-HBDH was set as a continuous variable, the results showed that α-HBDH was an independent risk factor for postoperative cardiac injury. In model 4, α-HBDH was set as a categorical variable; it was still an independent risk factor for postoperative cardiac injury. The risk for cardiac injury was 9.869-fold higher in the T3 group than in the TI group (Table 3).
Similarly, LDH was set as a continuous variable in the main study endpoint (in-hospital mortality), and the four models were adjusted respectively. The results showed that LDH was not an independent risk factor for in-hospital mortality after adjustment of model 4. LDH was then set as a categorical variable, and patients were divided into three groups (T1, T2, and T3). Following adjustments of model 4, the results showed that LDH was not an independent risk factor for in-hospital mortality. Regarding the secondary endpoint (cardiac injury), when LDH was set as a continuous variable and after adjustments of models 1-4, the results showed that LDH was not an independent risk factor for postoperative cardiac injury. Meanwhile, when LDH was set as a categorical variable, the results showed that it was an independent risk factor for postoperative cardiac injury in models 1 and 2, with model 2 as an example and T1 as the reference group. The risk of cardiac injury was 2.741-fold higher in the T3 group than in the T1 group. However, after adjustments of models 3 and 4, LDH was not an independent risk factor for postoperative cardiac injury (Table 3). The above results show that α-HBDH is more specific than LDH in predicting hospital death and cardiac injury.
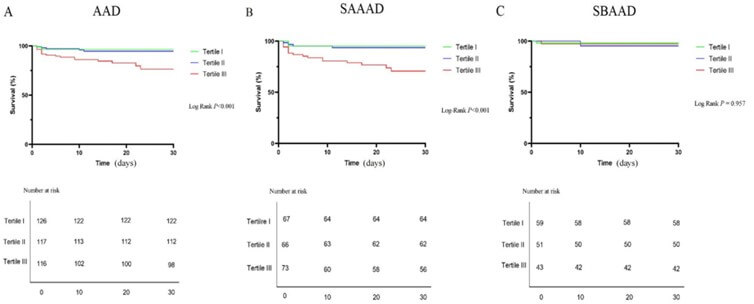
3.4. Survival Analysis of the Relationship between α-HBDH and in Hospital Mortality
As shown in (Figure 4A), high levels of α-HBDH on admission were associated with a poor survival rate within 30 days after surgery for patients with AAD (log rank, P<0.01). Patients were further classified into acute stanford A (n = 216) and acute stanford B (n = 153) groups. Although there was no significant correlation between α-HBDH and the acute stanford B group (log rank, P > 0.05; Figure 4B), increased α-HBDH levels were significantly associated with a lower survival rate within 30 days after surgery in the acute stanford A group (log rank, P<0.01; Figure 4C).
3.5. RCS Analysis for the Prediction Model
After adjusting for age, gender, levels of AST, ALT, Cr, glucose, and LDH, and history of cardiac injury with 5%, 35%, 65%, and 95% nodes in the prediction model, the results of RCS analysis showed that there was a positive linear relationship between α-HBDH and in-hospital mortality (P = 0.543). When the level of α-HBDH was low, the correlation between α-HBDH and in-hospital mortality did not change. However, when the level of α-HBDH increased, the correlation between α-HBDH and in-hospital mortality showed an increasing trend (P<0.001). Notably, when the α-HBDH level was <204 U/L (the cut-off point) and >204 U/L, the left and right hazard ratios were <1 and >1, respectively (P<0.05), suggesting that patients with an α-HBDH level >204 U/L on admission have an increased risk for in-hospital mortality and a poor prognosis (Figure 5A).
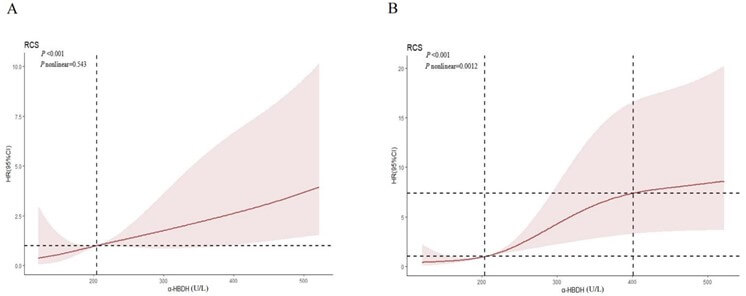
The relationship between α-HBDH and postoperative cardiac injury was analyzed by the prediction model that adjusted for the abovementioned factors and by RCS. The results showed that there was a non-linear relationship between α-HBDH and cardiac injury (P=0.0012). The risk for cardiac injury was lower when the α-HBDH level was <204 U/L but increased remarkably when the α-HBDH level was >204 U/L; the risk stabilized when the α-HBDH level reached 401 U/L. In summary, α-HBDH level >204 U/L increases the risk of in-hospital mortality and postoperative cardiac injury (Figure 5B).
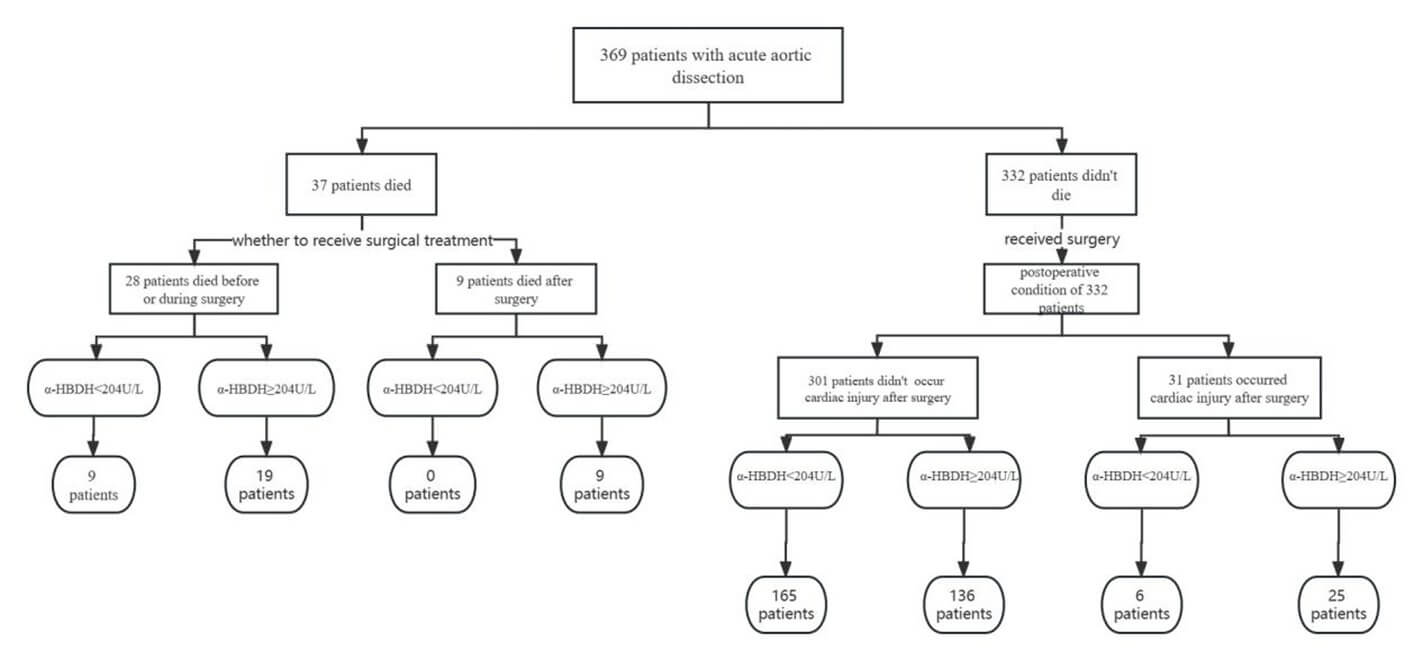
3.6. Flow Chart of Patient Origin and α-HBDH Levels
Among the 369 patients with AAD, 37 died; 28 died either without undergoing surgery or intraoperatively due to rapid progression of the disease. Among these patients, 19 and 9 patients had high and low α-HBDH levels. Additionally, nine patients died after surgery, all of whom had high α-HBDH levels. These results suggest that elevated α-HBDH levels increase the risk of mortality with or without surgery. Among the 332 patients who underwent surgery, it was found that surgery may be a potential risk factor for the development of complications. In this cohort, 31 patients developed cardiac injury after surgery; 25 of whom had high α-HBDH levels. This shows that the influence of elevated α-HBDH levels before surgery may also predict the postoperative prognosis. Thus, it was suggested that preoperative intervention and intraoperative protection of the myocardium should be performed for patients with AAD and high α-HBDH levels to reduce the incidence of adverse postoperative cardiac events (Figure 6).
3.7. Subgroup Analysis
We used age, gender, hypertension, smoking, drinking, type of AAD (stanford), and AST, Cr, and LDH levels as stratification variables to observe the trends of effect sizes in these variables (Table 4). We noted that none of the interactions were observed based on the primary observation endpoint (in-hospital mortality) through our a priori specification (all P>0.05), demonstrating that the relationship between α-HBDH and in-hospital mortality was stable. Similarly, based on the secondary end point (cardiac injury), we found no interaction (all P>0.05), demonstrating that the relationship between α-HBDH and cardiac injury was also stable.
TABLE 4: Results of subgroup analysis and interaction
analysis.
|
Characteristic |
Total n |
<204U/L n (%) |
≥204U/L n (%) |
In-hospital mortality |
P for interaction |
Cardiac injury |
P for interaction |
|
OR (95% CI) P |
OR (95% CI) P |
||||||
|
Age (years) |
|
|
|
|
0.714 |
|
0.685 |
|
< 60 |
249 |
112(62.2) |
137(72.5) |
3.446(1.243,9.549) 0.017 |
|
0.748(0.369,1.518) 0.422 |
|
|
≥ 60 |
120 |
68(37.8) |
52(27.5) |
3.349(0.970,11.567) 0.056 |
|
1.967(0.604,6.404) 0.261 |
|
|
Gender |
|
|
|
|
0.353 |
|
0.421 |
|
Male |
273 |
43(23.9) |
53(28.0) |
4.287(1.552,11.843) 0.005 |
|
1.149(0.574,2.299) 0.695 |
|
|
Female |
96 |
137(76.1) |
136(72.0 |
1.994(0.569,6.990) 0.280 |
|
0.647(0.192,2.177) 0.482 |
|
|
Hypertension |
|
|
|
|
0.818 |
|
0.558 |
|
No |
99 |
46(25.6) |
53(28.0) |
2.809(0.538,14.657) 0.221 |
|
1.300(0.425,3.978) 0.646 |
|
|
Yes |
270 |
134(74.4) |
136(72.0) |
3.501(1.442,8.503) 0.006 |
|
0.874(0.425,1.796) 0.714 |
|
|
Smoking |
|
|
|
|
0.799 |
|
0.571 |
|
No |
172 |
76(42.2) |
96(50.8) |
3.600(1.150,11.267) 0.028 |
|
0.821(0.341,1.980) 0.661 |
|
|
Yes |
197 |
104(57.8) |
93(49.2) |
2.933(0.992,8.671) 0.052 |
|
1.165(0.510,2.659) 0.778 |
|
|
Drinking |
|
|
|
|
0.268 |
|
0.197 |
|
No |
163 |
75(41.7) |
88(46.6) |
2.176(0.792,5.980) 0.132 |
|
0.625(0.258,1.567) 0.325 |
|
|
Yes |
206 |
105(58.3) |
101(53.4) |
5.471(1.522,19.665) 0.009 |
|
1.414(0.626,3.190) 0.404 |
|
|
Type of
AAD |
|
|
|
|
0.788 |
|
0.374 |
|
A |
216 |
99(55.0) |
117(61.9) |
3.250(1.397,7.558) 0.006 |
|
0.778(0.363,1.667) 0.518 |
|
|
B |
153 |
81(45.0) |
72(38.1) |
2.286(0.203,25.751) 0.503 |
|
1.368(0.511,3.665) 0.533 |
|
|
AST (μ/L) |
|
|
|
|
0.194 |
|
0.064 |
|
Normal (≤40) |
240 |
142(78.9) |
98(51.9) |
4.362(1.347,14.131) 0.014 |
|
1.133(0.538,2.389) 0.742 |
|
|
High (> 40) |
129 |
38(21.1) |
91(48.1) |
1.516(0.516,4.457) 0.449 |
|
0.770(0.277,2.141) 0.617 |
|
|
Cr (μmol/L) |
|
|
|
|
0.533 |
|
0.456 |
|
Normal (70-100) |
131 |
64(35.6) |
67(35.4) |
1.210(0.310,4.722) 0.784 |
|
1.800(0.640,5.066) 0.266 |
|
|
High (> 100) |
107 |
36(20.0) |
71(37.6) |
2.106(0.711,6.234) 0.179 |
|
0.487(0.166,1.433) 0.191 |
|
|
LDH (U/L) |
|
|
|
|
0.427 |
|
0.135 |
|
Normal (109-245) |
193 |
89(49.4) |
104(55) |
2.562(1.073,6.114) 0.034 |
|
1.246(0.454,3.422) 0.669 |
|
|
High (> 245) |
175 |
91(50.6) |
84(44.4) |
8.182(0.985,67.978) 0.052 |
|
0.920(0.427,1.983) 0.832 |
|
4. Discussion
The main findings of this study show that i) α-HBDH is independently associated with in-hospital mortality and cardiac injury in patients with AAD; ii) α-HBDH is more specific than LDH for predicting postoperative cardiac injury in patients with AAD; iii) high levels of α-HBDH on admission were associated with a poor survival rate within 30 days after surgery, especially in patients with SAAAD; iv) the α-HBDH level was linearly correlated with in-hospital mortality and non-linearly correlated with postoperative cardiac injury, with a cut-off value of 204 U/L; and 5) the association between α-HBDH and in-hospital death and cardiac injury was stable. To the best of our knowledge, this was the first study to reveal a potential correlation between α-HBDH and clinical outcomes as well as complications in other organ systems in patients with AAD.
Since the underlying mechanisms of the occurrence and development of spontaneous AAD remain unclear, screening of AAD and prevention of death of patients have been restricted [16]. Previous reports have shown that preoperative levels of D-dimer, Cr, uric acid, serum tenascin-C, and inflammatory factors are associated with short-term mortality in AAD [17, 18]. Additionally, malperfusion in multiple organs and disturbances in hemodynamics, including hypotension, shock, cardiac tamponade, insufficient pulse, and renal failure, are also associated with short-term mortality [19]. Among these indicators, an increased D-dimer level reflects the risk of thrombosis and bleeding, and elevated levels of inflammatory factors indicate local or systemic inflammation, all of which are relevant in determining the status and prognosis of patients with AAD.
LDH is a key enzyme of anaerobic metabolism and a functional checkpoint for glucose restoration during gluconeogenesis and single-stranded DNA metabolism [20]. LDH consists of five isozymes with different combinations of H and M subunits: LDH1 (H4), LDH2 (H3M), LDH3 (H2M2), LDH4 (HM3), and LDH5 (M4). Activity of α-HBDH is highest in the myocardium and lowest in the liver and skeletal muscles [21-25]. Since α-HBDH activity may better reflect LDH1 and LDH2, which accounts for approximately 90% of the total LDH in the human myocardium, the specificity of elevated α-HBDH levels may be better than LDH for predicting cardiac injury in patients with AAD [26]. He, et al. [10] recently demonstrated that the high levels of LDH were positively associated with in-hospital mortality in patients with AD. In this study, α-HBDH was positively correlated with LDH. Additionally, α-HBDH levels were lower than LDH levels when death and cardiac injury occurred, indicating that α-HBDH was more sensitive than LDH in this aspect.
According to our results, serum α-HBDH levels may diagnose and predict whether patients with AAD would develop cardiac complications postoperatively, which contributes to short-term mortality. Meanwhile, sharp increases in α-HBDH levels indicate the severity of cardiac injury. The main strength of this study was that we identified a novel biomarker that is associated with postoperative cardiac injury in patients with AAD, providing an important supplement for the index of cardiac enzymes that previously only focused on LDH. More importantly, α-HBDH showed a higher specificity for predicting cardiac complications than LDH. There are several reasons for postoperative cardiac injury, for except different degrees of cardiac injury caused by preoperative heart-related complications, surgical intervene could also lead to postoperative cardiac adverse events, so α-HBDH as an indicator to predict the risk of postoperative cardiac damage will reflect the possible consequences of these factors well. Preoperative and intraoperative protection of the myocardium may be performed based on α-HBDH levels to reduce the risk of death and incidence of adverse postoperative cardiac events in patients with AAD.
Our study has some strengths: i) our sample size is relatively large; ii) this study is an observational study, and it is susceptible to potential confounders; however, we used strict statistical adjustments to minimize residual confounders; and iii) we handled the target independent variables as both continuous and categorical variables, which can reduce the contingency in data analysis and enhance the robustness of the results.
5. Conclusion
α-HBDH levels in patients with AAD on admission were independently associated with in-hospital mortality and cardiac injury, especially in adverse postoperative cardiovascular events. α-HBDH is superior to LDH in predicting cardiac injury in patients with acute aortic dissection α-HBDH levels on admission may be used to identify high-risk patients with AAD and those with a poor prognosis.
Study Limitations
Some limitations of this study must be mentioned. We only made a comparison between α-HBDH and LDH, and did not make a comparison between α-HBDH and other myocardial enzymes such as CK-MB, BNP, etc. The association between α-HBDH and in-hospital mortality and short-term prognosis of patients with AAD was evaluated in single-center; the predictive efficacy of α-HBDH on the long-term prognosis of these patients should be explored. Also, it would be beneficial to reveal the underlying mechanisms by dynamically measuring the α-HBDH level. Moreover, other factors that correlate with in-hospital mortality should be considered, including the effects of different operative methods on cardiac injury as well as adverse effects of long-term cryogenic circulation and other procedures during treatment. Finally, as a single-center study, the results must be interpreted with caution when extrapolating them into other settings.
Conflicts of Interest
None.
Funding
Ma Dong received funding from National Natural Science Foundation of China (No.81700416 and No.82270508), Natural Science Foundation of Hebei Province (H2022206279), Key Laboratory of Neural and Vascular Biology, Ministry of Educational foundation (NV20210006), and Science and Technology Research Project of Colleges and Universities of Hebei Province (QN2022164). Yong-bo Zhao is funded by 2022 Hebei Provincial Medical Science Research Major Projects (No. 20221293).
Acknowledgements
We also thank all staff participation from the Cardiac Surgery Department, the Forth Hospital of Hebei Medical University. Some of the icons in the graphic abstract were created by designer from (Link) the authors also thank them for their contributions.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
[1] Christoph A Nienaber, Rachel E
Clough, Natzi Sakalihasan, et al. “Aortic dissection.” Nat Rev Dis Primers,
vol. 2, pp. 16053, 2016. View at: Publisher
Site | PubMed
[2] Firas F Mussa, Joshua D Horton,
Rameen Moridzadeh, et al. “Acute aortic dissection and intramural hematoma: A
systematic review.” JAMA, vol. 316, no. 7, pp. 754-763, 2016. View at: Publisher Site | PubMed
[3] Christian Smedberg, Johnny Steuer,
Karin Leander, et al. “Sex differences and temporal trends in aortic
dissection: a population-based study of incidence, treatment strategies, and
outcome in Swedish patients during 15 years.” Eur Heart J, vol. 41, no.
26, pp. 2430-2438, 2020. View at: Publisher
Site | PubMed
[4] Arminder S Jassar, Thoralf M Sundt
3rd “How should we manage type A aortic dissection?” Gen Thorac Cardiovasc
Surg, vol. 67, no. 1, pp. 137-145, 2019. View at: Publisher Site | PubMed
[5] Thomas T Tsai, Arturo Evangelista,
Christoph A Nienaber, et al. “Long term survival in patients presenting with
type A acute aortic dissection: insights from the International Registry of
Acute Aortic Dissection (IRAD).” Circulation, vol. 114, no. 1
Suppl, pp. 350-356, 2006. View at: Publisher
Site | PubMed
[6] Sebastian Pagni, Brian L Ganzel,
Jaimin R Trivedi, et al. Early and Midterm Outcomes Following Surgery for Acute
Type A Aortic Dissection. J Card Surg, vol. 28, no. 5, pp. 543-549,
2013. View at: Publisher Site | PubMed
[7] Patrick Ohlmann, Laurent Jaquemin,
Olivier Morel, et al. “Prognostic value of C-reactive protein and cardiac
troponin I in primary percutaneous interventions for ST-elevation cardiac
infarction.” Am Heart J, vol. 152, no. 6, pp. 1161-1167, 2006. View at: Publisher Site | PubMed
[8] R Dissmann, T Linderer, R Schröder
“Estimation of enzymatic infarct size: direct comparison of the marker enzymes
creatine kinase and alpha-hydroxybutyrate dehydrogenase.” Am Heart J,
vol. 135, no. 1, pp. 1-9, 1998. View at: Publisher Site | PubMed
[9] G Karayalcin, P Lanzkowsky, A B Kari
“Serum alpha-hydroxybutyrate dehydrogenase levels in children with sickle cell
disease.” Am J Pediatr Hematol Oncol, vol. 3, no. 2, pp. 169-171, 1981. View at: Publisher Site | PubMed
[10]
Huaping
He, Xiangping Chai, Yang Zhou, et al. “Association of lactate dehydrogenase
with in-hospital mortality in patients with acute aortic dissection: A
retrospective observational study.” Int J Hypertens, vol. 2020, pp.
1347165, 2020. View at: Publisher
Site | PubMed
[11] Yingchao Zhu, Yaodan Bi, Yabing
Zhang, et al. “Preoperative serum alpha-hydroxybutyrate dehydrogenase level as
a predictor of postoperative mortality and morbidity after noncardiac surgery:
A propensity-adjusted analysis.” Surgery, vol. 171, no. 4, pp.
1027-1035, 2022. View at: Publisher Site | PubMed
[12]
A
van der Laarse, W T Hermens, L Hollaar, et al. “Assessment of cardiac damage in
patients with acute cardiac infarction by serial measurement of serum
alpha-hydroxybutyrate dehydrogenase levels.” Am Heart J, vol.
107, no. 2, pp. 248-260, 1984. View at: Publisher Site | PubMed
[13] “Section 2: AKI Definition.” Kidney
Int Suppl (2011),
vol. 2, no. 1, pp. 19-36, 2012. View at: Publisher
Site | PubMed
[14]
Norbert
H Lameire, Adeera Levin, John A Kellum, et al. “Harmonizing acute and chronic
kidney disease definition and classification: report of a Kidney Disease:
Improving Global Outcomes (KDIGO) Consensus Conference.” Kidney Int,
vol. 100, no. 3, pp. 516-526, 2021. View at: Publisher
Site | PubMed
[15]
Lakhmir
S Chawla, Rinaldo Bellomo, Azra Bihorac, et al. “Acute kidney disease and renal
recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16
Workgroup.” Nat Rev Nephrol, vol. 13, no. 4, pp. 241-257, 2017. View at: Publisher Site | PubMed
[16]
Toshihiro
Fukui “Management of acute aortic dissection and thoracic aortic rupture.” J
Intensive Care, vol. 6, pp. 15, 2018. View at: Publisher Site | PubMed
[17]
Arturo
Evangelista, Eric M Isselbacher, Eduardo Bossone, et al. “Insights from the
international registry of acute aortic dissection: A 20-year experience of
collaborative clinical research.” Circulation, vol. 137, no. 17, pp.
1846-1860, 2018. View
at: Publisher
Site | PubMed
[18]
Joanna
Gawinecka, Felix Schönrath, Arnold von Eckardstein, et al. “Acute aortic
dissection: pathogenesis, risk factors and diagnosis.” Swiss Med Wkly,
vol. 147, pp. w14489, 2017. View at: Publisher
Site | PubMed
[19]
Fulvio
Morello, Anna Ravetti, Peiman Nazerian, et al. “Plasma lactate dehydrogenase levels
predict mortality in acute aortic syndromes: A diagnostic accuracy and
observational outcome study.” Medicine (Baltimore), vol. 95, no. 6, pp.
e2776, 2016. View
at: Publisher
Site | PubMed
[20]
Giuseppina
Laganá, Davide Barreca, Antonella Calderaro, et al. “Lactate dehydrogenase
inhibition: biochemical relevance and therapeutical potential.” Curr Med
Chem, vol. 26, no. 18, pp. 3242-3252, 2019. View at: Publisher
Site | PubMed
[21]
R
P MACDONALD, J R SIMPSON, E NOSSAL “Serum lactic dehydrogenase: a diagnostic
aid in cardiac infarction.” J Am Med Assoc, vol. 165, no. 1, pp. 35-40,
1957. View at: Publisher Site | PubMed
[22]
H
T WILSON, J A LAZARONI Jr, E C MAIER “A test for cardiac infarction; the
present status of the use of serum lactic acid dehydrogenase.” Calif Med,
vol. 88, no. 5, pp. 369-371, 1958. View at: PubMed
[23]
B
A ELLIOTT, J H WILKINSON “Serum "alpha-hydroxybutyric dehydrogenase"
in cardiac infarction and in liver disease.” Lancet, vol. 1, no.
7179, pp. 698-699, 1961. View at: Publisher
Site | PubMed
[24]
S
B ROSALKI, J H WILKINSON “Serum alpha-hydroxybutyrate dehydrogenase in
diagnosis.” JAMA, vol. 189, pp. 61-63, 1964. View at: Publisher Site | PubMed
[25] E F Roth Jr, P A Bardfeld, S J Goldsmith, et al. “Sickle cell crisis as evaluated from measurements of hydroxybutyrate dehydrogenase and myoglobin in plasma.” Clin Chem, vol. 27, no. 2, pp. 314-316, 1981. View at: PubMed
[26] S B ROSALKI “Serum alpha-hydroxybutyrate dehydrogenase: a new test for cardiac infarction.” Br Heart J, vol. 25, no. 6, pp. 795-802, 1963. View at: Publisher Site | PubMed
