Received: Mon 07, Aug 2023
Accepted: Mon 21, Aug 2023
Abstract
Hydatid is a global disease and affects almost all organs of the body, liver being the most common. Patients with hydatid disease often present as a therapeutic challenge since there is no specific consensus regarding the optimal management. The complete therapy of hydatid disease should aim to eliminate the parasite to cure the disease. The first and important step should be prevention of the disease by implementation of safe hygienic practices. Medical and surgical management go hand in hand with surgery being the treatment of choice, controversy still exists about the most appropriate surgical approach. Surgical management remains the gold standard for liver hydatid cyst, however individualization of treatment modality depending upon the type, number, location, recurrent disease and complications is recommended.
Keywords
Hydatid cyst, echinococcosis, pericystectomy, hepatectomy, PAIR
1. Introduction
Hydatid disease of the liver is a zoonotic parasitic infection caused by larval forms of the tapeworm Echinococcus granulosus. Hydatid cysts may involve any organ of the human body, most frequently the liver (60-70%) and the lungs (20-30%) [1]. Hydatid cyst disease has been known since times of galen and hippocrates. First described by Thebesius in 17th Century, the disease was named as hydatid cyst by Rudolphi in 1808 [2, 3]. The disease remains endemic in sheep raising areas of world including Kashmir valley. Dogs are the definitive hosts for completion of lifecycle of Echinococcus granulosus and sheep being the major intermediate hosts; man is only incidentally infected. Liver hydatid cysts are often diagnosed incidentally with 75% being asymptomatic [4].
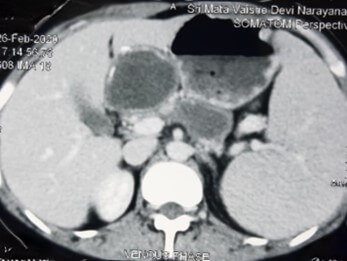
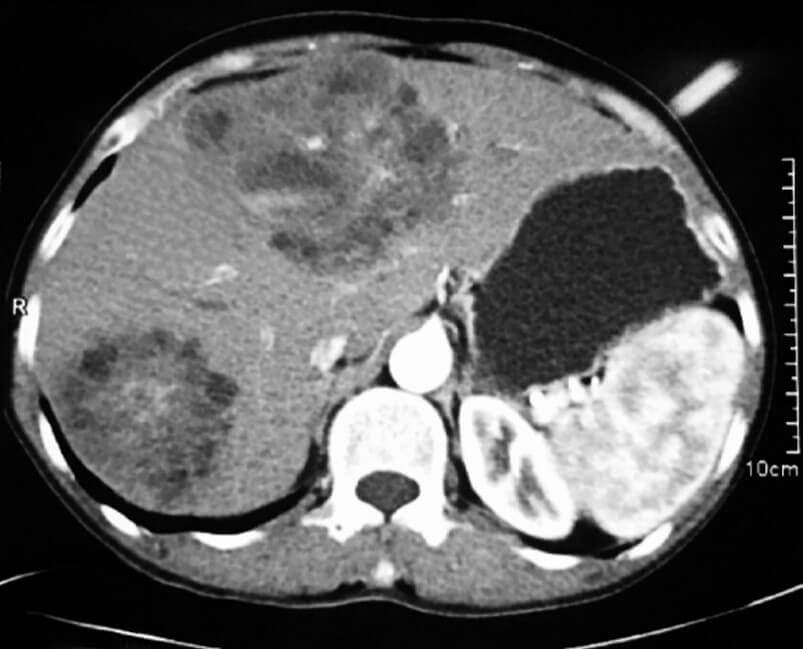
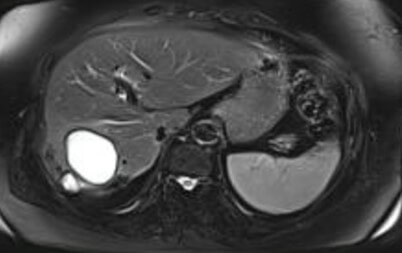
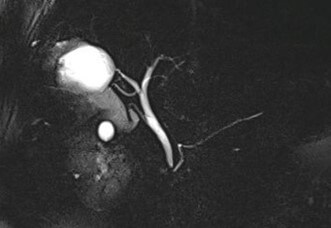
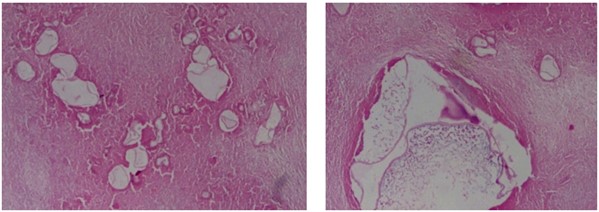
Based on USG classification of Gharbi et al. [5], the hydatid cysts are classified into five categories: type I- pure fluid collection; type II- fluid collection with septa (Honey comb appearance); type III- fluid collection with split wall (Water Lily sign); type IV- heterogenous; and, type V- reflecting thick walls. Following the WHO classification five major cyst types of hydatid cysts have been recognized [6]: CE1– CE5, characterized by the appearance of the contents of the cyst and cyst wall. Type CE1 (unilocular, simple cysts) and type CE2 (multivesicular or multiseptated cysts) is considered as active as they are likely to contain viable protoscoleces. Type CE3 (unilocular cysts with detached laminated membrane) are considered as transitional representing the beginnings of cyst degeneration. Type CE4 (heterogeneous or hyperechoic degenerative contents) and type CE5 (calcified cysts) (Figure 1) are inactive, as the parasite is considered to be of low viability [7]. Alveolar echinococcosis is a distinct, rare zoonotic disease caused by Echinococcus multilocularis which present as hepatic pseudotumors (Figure 2). CT and MRI are helpful in diagnosis and surgical planning. MRI is useful to delinate cystobiliary communications preoperatively (Figures 3 & 4).
Patients with hydatid cysts frequently present as a therapeutic challenge to the clinician as there is no consensus as to the optimal form of treatment of hydatid cyst. The ideal therapy of the hepatic hydatid should aim to cure the disease by eliminating the parasite. Three therapeutic modalities are currently into practice for treatment of hepatic cystic echinococcosis: chemotherapy, surgery, and percutaneous drainage, with the latter two being performed with or without chemotherapy. Surgical treatment of hydatid cyst typically involved an open approach with meticulous operative site packing, employing a variety of conservative and radical operative techniques. The initial and most important step to prevent hydatid disease is implementation of basic hygienic practices in daily life.
The surgical treatment of hepatic hydatid varies from complete resection to minimally invasive procedures like percutaneous aspiration [8]. Surgical treatment either open or laparoscopic has traditionally been the mainstay of the therapy for hepatic hydatidosis. Although surgical intervention is the treatment of choice, controversy still exists about the most appropriate surgical approach [9-12]. Surgical modalities remain the gold standard for treatment of hydatid cysts of liver as it allows for direct removal of the active parasite coupled with addressal of the residual cavity, preservation of normal liver parenchyma and prevention of recurrence. Individualization of treatment modality for patients according to the number and location of cysts, the presence of cyst infection, and complications is recommended [13].
2. Antiparasitic Chemotherapy
2.1. Benzimidazoles
Mebendazole is a benzimidazole carbamate agent with an in vivo activity against hydatid disease. The drug interferes with the mechanism of glucose absorption through the parasite wall leading to depletion of glycogen and subsequent degenerative changes in mitochondria and endoplasmic reticulum of the germinal cells. Albendazole is a more active agent with improved gastrointestinal absorption and bio-availability [14, 15]. Both drugs may lead to decrease in the size of hydatid cysts and sterilization of cyst contents. However, clinical and radiological resolution is unpredictable without drainage and may occur in less than half of treated patients [16]. The usual dose of orally administered albendazole is 10-15 mg/kg/day in two divided doses or as a fixed dose of 400 mg twice daily. Mebendazole is used at a dose of 40-50 mg/kg in three divided doses. Treatment of hepatic cystic echinococcosis with benzimidazoles alone is not as effective as compared to combined approach using chemotherapy and drainage. Benzimidazole therapy is typically administered as 1-6 monthly cycles separated by a 10-14 day drug free intervals.
2.2. Praziquantel
It is a third anti-parasitic agent, has a limited use in treatment of hepatic hydatid cysts. Praziquantel is an iso-quinolone derivative which increases the calcium permeability of the parasite cell membrane resulting in strong contractions and paralysis of the musculature of the parasite, leading to detachment from host tissue [17]. Praziquantel is also effective as an in vitro scolicidal agent. It has a favorable pharmacokinetics when given at a dose of 50 mg/kg once weekly or biweekly.
3. Percutaneous Drainage
The availability of chemotherapeutic agents with a significant anti-echinococcal activity has made it possible to undertake transhepatic percutaneous drainage of hydatid cysts. Percutaneous treatment of hydatid cysts satisfies all the goals of surgery but substitutes the surgical removal of cyst by sclerosing and separation of the germinal membrane using scolicidal agents.
i) PAIR (Puncture, Aspiration, Injection and Re-aspiration): Patients undergoing PAIR therapy are also treated by peri-procedural oral benzimidazole (albendazole or mebendazole) for seven days before and 28 days after drainage, though series of PAIR without drug therapy have been reported [18, 19]. However, concomitant pre- and post- interventional chemotherapy with albendazole/mebendazole offers the advantage of reducing risk of disease recurrence and intraperitoneal seeding of infection.
ii) PEVAC (Percutaneous Evacuation): A safe and effective method for percutaneous treatment of multi-vesicular hydatid cysts with or without cystobiliary communications containing non-drainable material.
iii) Modified catheterisation technique (MoCat).
iv) Dilatable Multifunction Trocar (DMFT).
These Non-PAIR percutaneous modalities are generally reserved for multi-vesicular cysts or cysts with predominantly solid component that are difficult to drain or tend to recur after PAIR therapy.
4. Surgical Options
All patients with symptomatic hydatid cysts and those with asymptomatic cysts >5 cm in size are candidates for surgery. The aim of the surgical procedure is to remove all living parasites and daughter cysts [20]. The principles of surgery in hepatic hydatidosis are inactivation of the cestode parasites, evacuation of the cyst cavity, removal of the germinal layer, and obliteration of the residual cavity. Surgery remains the gold standard and primary treatment of hydatid cyst of liver with a variety of techniques based on the principles of eradication and elimination of recurrence while avoiding spillage [21]. Although surgery is considered the treatment of choice for hydatid disease of the liver, controversies regarding the preferred operative technique, management of the residual cavity and the use of scolicidal agents are still existent in modern practice.
Surgical options for the management of hepatic hydatid cysts consist of conservative, radical and laparoscopic approaches. Conservative surgical techniques include simple tube drainage, marsupialization (surgical exteriorization of a cyst by resection of the anterior wall and suture of the cut edges of the remaining cyst to the adjacent edges of the skin), capitonnage (surgical closure of the cyst cavity and approximation of the opposite surfaces by applying sutures), deroofing, partial simple cystectomy, open or closed total cystectomy with or without omentoplasty. Radical surgery for hydatid liver includes total pericystectomy, partial hepatectomy or lobectomy. In the last decade, a shift towards radical surgical procedures in management of hepatic hydatid cysts appears to result in lesser postoperative complications, fewer relapses and lower mortality than conservative techniques [22, 23]. Paralleling the progress in minimal access surgery, laparoscopic treatment of hydatid cyst liver has achieved increasing popularity [24]. Laparoscopic drainage of hepatic hydatid cyst is a safe and effective minimally invasive surgical technique [25, 26].
Irrespective of the surgical technique planned, a benzimidazole agent used as an adjunct to surgery in an attempt to sterilize the cyst contents and reduce the risk of anaphylaxis and dissemination is preferred [27]. Packing of the operative field meticulously using packs soaked with scolicidal agents that kill the scoleces and the protoscolices of the parasite residing within the hydatid cyst, or potentially leaking from the cyst during surgical manipulation is necessary irrespective of the surgical technique employed. Various scolicidal agents used in surgical (and PAIR) approaches include: hypertonic saline, povidone iodine, hydrogen peroxide, formalin, silver nitrate, and albendazole, which can be used alone or in combination. The type of scolicidal agent by itself does not significantly influence the outcome of the surgical or non-surgical intervention, but different solutions have their advantages and relative contraindications.
Laparoscopic treatment of hydatid cyst liver is gaining popularity [28]. Laparoscopic surgery offers lower morbidity and shorter hospital stay with faster surgery [29] in addition to advantage of better visualization of the cyst cavity under magnification, which allows better detection of inconspicuous bile leaks [30]. Although the length of hospital stay following laparoscopic surgery is generally shorter and morbidity rates lower than for open procedures [25], however laparoscopy has a theoretical dis-advantage of spillage of the parasite under the high intra-abdominal pressure due to pneumoperitoneum. Several operative techniques and instruments have been devised for the complete removal of the germinative membrane of hydatid cyst using laparoscopic approach. Use of an aspirator-grinder apparatus by Alper et al. [31] and large-bore suction catheter by bickel and eitan has been recommended. In recent years, laparoscopic approach has been developed using an umbrella trocar to perform a partial or total cystectomy [30]. Conversion to open technique has been reported in 23% to 27% cases [30, 32].
Conservative surgical options for hydatid cysts of liver include unroofing of the cyst associated with management of residual cavity using various procedures. In conservative operations, the contents of the cyst are evacuated without removal of the pericyst. Conservative procedures are technically simple, safe and are useful in the management of uncomplicated hydatid cysts [33]. Morbidity after conservative operations is mainly related to the extent of cavity remaining after the contents of the cyst have been evacuated. Although being technically easier and safer, peri procedural morbidity is higher because of increased incidence of local recurrence and cavity related complications [34]. Bile leak from a cyst-biliary communication leading to external biliary fistulae, bilomas and biliary peritonitis are common cavity related complications (4%-28%) [37, 38]. Potential major complications associated with surgical treatment of hepatic hydatid cyst include postoperative haemorrhage, bile exudation from residual cavity, incisional biliary fistulae, cholangitis, wound infection, sepsis, pulmonary complications (pneumonia, pulmonary embolisation) and death. Topcu et al. reported that cysts > 10 cm in diameter have biliary system involvement in 22.2% of patients [39]. The indications for conservative surgery include elderly patients, high risk patients with high ASA levels, multiple cysts, cysts located deeply sized ≥ 10 cm, bilobar cysts and cysts located in the posterior segments of liver.
Radical operations remove the cyst completely along with the pericyst and include pericystectomy and hepatectomy (Figures 5-7). The most common radical technique used for management of hepatic hydatid cysts is total or partial cystectomy [10, 11]. As compared to conservative procedures radical methods have excellent results, but they are feasible in few cases. Whereas conservative procedures are relatively simple and still accepted, they are associated with a higher rate of morbidity. However, for complicated cysts, external drainage of the cystic cavity or unroofing procedure, partial pericystectomy and sometimes addition of omentoplasty are generally preferred. With advances in hepatobiliary surgery, a shift towards radical surgical interventions has been seen as they result in fewer postoperative complications, in particular recurrence, and lower mortality than the conservative procedures [22, 23]. The radical procedures have an increased operative risk when weighed for a benign disease but with a lower risk of recurrence [40]. A pericystectomy involves the excision of a cyst including the surrounding fibrous layer minimizes the risk of recurrence. Pericystectomy involves extensive dissection, resulting in a large wound area, which may lead to increased risk of bleeding or bile leaks. In patients with extensive parenchymal involvement, an anatomical liver resection provides the best option for definitive therapy. The rate of recurrence following surgery for hepatic hydatid disease has been reported to range from 1.1 to 9.6% [41, 42]. Radical surgery is recommended for younger patients, cysts located in the anterosuperior segments of liver and cysts in the left lateral segment, exophytic cysts, alveolar hydatid cyst and cysts ≥ 5 cm in diameter.
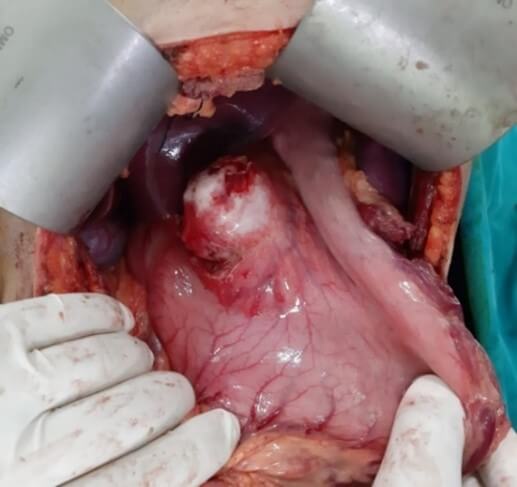
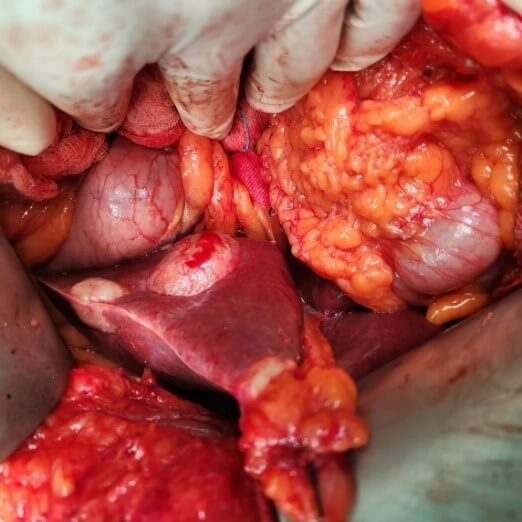
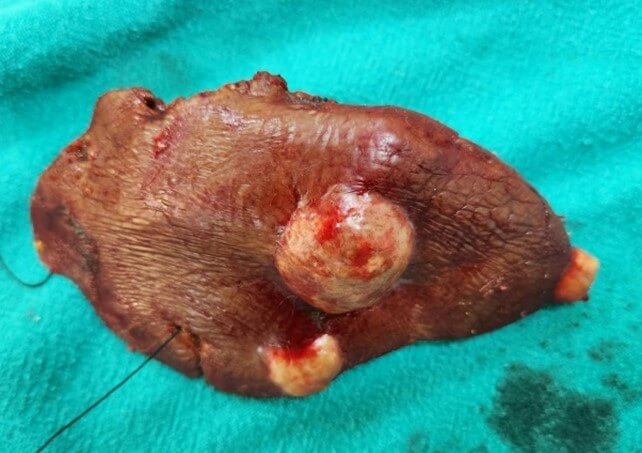
Yuksel et al. [43] in a randomised prospective study concluded that in comparison to conservative surgical approaches, radical surgical resection is an effective surgical option and prevents early local recurrence and cavity-related complications. Gollackner et al. [22] found that relapse rates are significantly lower in radical surgical procedures in contrast to conservative techniques. Similar conclusions have been derived by three retrospective, non- comparative studies, concluding that radical procedures are safe and efficient [44-46]. In a study comparing 102 patients undergoing radical surgery with 250 patients undergoing conservative surgical procedures, Tasev et al. [47] concluded that radical surgical procedures were associated with lower postoperative morbidity and mortality rates and a shorter postoperative hospital stay. Moumen et al. [48] concluded that patients treated conservatively by unroofing of the hydatid cyst of the liver are associated with a mortality of 1.3%, constituting a clear advantage of conservative procedures with regard to radical surgery.
4.1. Management of Residual Cavity
Management of the residual cavity following the evacuation of the contents of hydatid cysts remains controversial. Many techniques have been recommended for management of residual cavity following evacuation of cyst contents depending on the size and location of the cyst, the presence or absence of complications and the surgeon’s experience. Superficial, uncomplicated or unilocular cyst can be left open. Capitonnage can be accomplished by obliteration of the pericystic walls by absorbable suture material after excision of the redundant cyst cavity. Capitonnage is an effective option for deeply located cysts in liver, but it is difficult if the cyst wall is calcified and thickened. Omentoplasty can be achieved by placing a viable pedicle flap of omentum into the cyst cavity. Omentoplasty assists in healing of the raw surface and promotes the resorption of serosal fluid. It also helps macrophages to reach the septic foci.
Omentopexy is generally used for single, un-complicated hydatid cysts. It can also be combined with external drainage for infected cysts. Introflexion implies rolling the edge of the cyst and fixing it to the bottom of the cavity. In the presence of infection, external drainage achieved by placing a catheter into the cyst is indicated. However, the routine use of external tube drains after partial resection of liver hydatid cyst leads to increased postoperative morbidity and prolonged hospital stay.
4.2. Scolicidal Agents
Over the past years, hypertonic saline and cetrimide have become the scolicidal agents of choice. Lowest concentration of saline used for scolicidal purpose should be 20% and one it should be avoided in patients having cysto-biliary communication because of the risk caustic sclerosing cholangitis [49]. Although it was demonstrated that 5% saline has no scolicidal effect, many surgeons recommend the use of 3% saline [50].
Studies have been published on the use of different concentrations of scolicidal agents with different contact time for the development of complications such as recurrence and postoperative cholangitis. The scolicidal agents that have been researched are hypertonic saline (3%-30% for 5-30 min), povidone-iodine (10%), chlorhexidine (0.04%-4%), cetrimide (0.1%-0.4%), ethyl alcohol (95%), hydrogen peroxide (3%) and formalin. A study conducted by Besim et al. [51] compared the effects of different scolicidal agents at different concentrations and concluded that povidone iodine, 20% saline, 3% hydrogen peroxide, 95% ethyl alcohol, and 10% savlon solutions used for a contact period of 15 minutes inactivated the protoscolices. The re-infectivity was higher with agents using concentrations of < 20% and a contact time of < 5 minutes. Use of 20% and 30% hypertonic saline solutions have been reported to cause sclerosing cholangitis [52, 53].
4.3. Calcified Hydatid Cyst
The management of complicated hepatic hydatid cyst is generally difficult [54]. There is no consensus about the management of calcified cyst at present. Whether a calcified cyst is responsible for the patients symptoms, the question about surgical intervention in calcified cysts, treatment of asymptomatic patient with a calcified cyst, have not been answered [55]. About 10% to 16% of the cases of hepatic hydatid cyst undergo calcification which takes about 5 to 10 years to develop, commoner in the aged population [56]. Most of these calcified cysts are biologically and clinically silent. Rupture into the biliary tract, abdominal cavity or the thorax is not seen and they rarely become infected [57]. Some authors recommend that small calcified cysts less 5cm in diameter should not be treated surgically [55, 58]. Silent cysts more than 5 cm should best be left untreated. Some authors conclude that large cysts should be operated regardless the degree of calcification and the clinical picture.
4.4. Cyst-Biliary Communication
All efforts should be made intra-operatively to detect any cyst-biliary communications so as to reduce postoperative bile leaks. Use of toxic scolicidal agents like povidone-iodine should be avoided since they lead to interference with the identification of cyst-biliary communications. The residual cavity after evacuation of cyst contents should be meticulously inspected for any biliary communication. Placement a white laparotomy pad in the residual cavity after cyst evacuation and inspection of residual cavity for bile staining aids in identification of bile leaks. Injection of coloured dye into the biliary tree and inspection of the residual cavity for any staining can also be done to identify bile leaks. Compression of gallbladder along with clamping of common bile duct and watching for expression of bile into residual cavity can also aid in identification of biliary communication. As a last resort an intra operative cholangiogram can be undertaken in suspicious cases. All cyst-biliary communications should be identified and meticulously ligated. In patients with large cyst-biliary communications or when closure is unsatisfactory, biliary decompression should be performed. External biliary fistulae following liver hydatid cyst evacuation tend to close spontaneously, although sometimes endoscopic biliary drainage may be required.
In a review of 304 cases, spontaneous closure of all 10 external biliary fistulae occurred over a period of 2-4 months [59]. In another series, out of 12 patients with external biliary fistulae 7 fistulae closed spontaneously, the maximum time to closure being 38 days [60]. In patients with postoperative external biliary fistulae endoscopic management in the form of endoscopic sphincterotomy, with or without stenting or nasobiliary drainage, plays a key role [61, 62]. Endoscopic sphincterotomy reduces the high intra-biliary pressure and promotes early closure of fistulae even in the absence of distal biliary obstruction [63]. Bile leak is a common problem after conservative surgery for hepatic hydatid disease. Early endoscopic biliary decompression helps to hasten fistula closure and reduce morbidity in patients with high-output fistulae. In contrast, majority of patients with low-output fistulae close spontaneously with conservative management; however, early endoscopic intervention may be beneficial. When biliary leakage in the early postoperative period is ≥ 250 mL, performing endoscopic sphincterotomy or stenting within 3-5 days allows early hospital discharge, reduces re-operation rate, reduces morbidity associated with prolonged drain and reduces the costs and psychosocial problems associated with prolonged follow-up.
4.5. Recurrent Hydatid Cyst
One of the major problems in the management of hepatic liver disease is recurrence. Recurrent disease is defined as the appearance of new active cysts after therapy for intra- or extra- hepatic disease. Recurrent disease manifests as the re-appearance of live cysts at previously treated cyst location or the appearance of new cysts at a different site resulting from spillage during index procedure. The cause of local recurrence is considered to be due to therapeutic failure to achieve permanent control of the primary treated cyst. Local recurrence may occur after surgical or radiological intervention. Various studies report the incidence of recurrence following surgery for liver hydatid cyst to be 0% to 25% [64, 65]. Although following radical surgery the recurrence rates range from 0% to 4.65% [66] in contrast to recurrence rates ranging from 4.65% to 25% in those who undergo conservative surgery [66].
The possible theories explaining the cause of recurrence of hydatid disease include: i) inadequate cyst content removal; ii) intraoperative spillage of cystic liquid; iii) undetected cysts; iv) satellite lesions; and, v) re-infestation. The incidence of recurrent disease may be reduced by prevention of intraoperative spillage of protoscolices and usage of an effective scolicidal agent during the operation. The practices which have been used intraoperatively to avoid parasitic dissemination include injection of scolicidal solution into the hydatid cyst, washing the residual cavity with scolicidal solution, and packing the operative field with sponges soaked in scolicidal agents [7].
The choice of operation is important. Except for the experienced hepatic surgeon performing complex procedures for hepatic hydatid cyst, this should be a simple cystectomy. Cystectomy is the safest procedure and pericystectomy implies a non-anatomical liver resection through a distorted, compressed, non-anatomical liver. The advocates of pericystectomy claim a lower recurrence rate; though not yet well established. In presence of cyst-biliary communication, scolicidal agents must not be injected into the cyst because of the risk of potentially fatal complication of sclerosing cholangitis. Liver resection can seldom be justified for Echinococcus granulosus but is the preferred treatment indeed and currently the only chance of cure in Echinococcus granulosus.
5. Role of Liver Transplantation
Liver transplantation tends to be an accomplished and established option of providing the cure in late-stage hepatic alveolar echinococcosis [67]. However, the practicability of liver transplantation is restricted by the shortage of donor organs and the requirement of high surgical technique [68, 69]. Moreover, the fiscal burden of procedure and post operative immunosuppression is particularly high. In certain cases of unresectable hepatic alveolar echinococcus (AED), liver transplantation with resection and reconstruction of the major vessels, including resection of IVC and even the right atrium, may be the only radical option making it possible to provide satisfactory immediate and long-term results of the surgical treatment of patients otherwise apparently doomed to death. Patkowski et al. [70] concluded in retrospective study cohort that liver transplantation was performed in 22 patient with echinococcal disease, including 4 patients who had undergone initial non-transplant surgery. Post-transplantation survival rates in their study group were 90%, 85%, and 75% at 1, 5, and 10 years, respectively. These surgeries are advisable to be performed only in highly specialized centers expertise with surgical hepatology and liver transplantation.
6. Conclusion
In conclusion, the most effective method for preventing postoperative recurrences in hepatic hydatid disease is radical surgery. The indications of surgery need to be customized in terms of age, medical history, location and size of the cyst, and relation of the cyst to the vasculature and biliary tree. Surgery combined with medical treatment with albendazole is effective in the eradication of hepatic hydatid disease and in the prevention of recurrence.
Conflicts of Interest
None.
REFERENCES
[1] A Menezes da Silva “Hydatid cyst of
the liver-criteria for the selection of appropriate treatment.” Acta Trop,
vol. 85, no. 2, pp. 237-242, 2003. View at: Publisher Site | PubMed
[2] Varela A, Burgos R, Castedo E
“Parasitic diseases of the lung and pleura. In: Patterson GA, Cooper JD,
Deslauriers J (eds): Pearson’s thoracic & esophageal surgery.” 3rd ed. Philadelphia:
Churchill Livingstone, pp. 550-565, 2008.
[3] Harlaftis NN, Aletras HA, Symbas PN
“Hydatid disease of the lung. In: Shields TW, Locicero III J, Reed CE, Feins RH
(eds) General thoracic surgery.” 7th ed. Philadelphia: Lippincott Williams
& Wilkins, pp. 1187-1195, 2009.
[4] M A Tolga Muftuoglu, N Koksal, U
Topaloglu “The role of omentoplasty in the surgical management of remnant
cavity in hepatic hydatid cyst.” HPB (Oxford), vol. 7, no. 3, pp.
231-234, 2005. View at: Publisher
Site | PubMed
[5] H A Gharbi, W Hassine, M W Brauner,
et al. Ultrasound examination of the hydatic liver. Radiology, vol. 139,
no. 2, pp. 459-463, 1981. View at: Publisher Site | PubMed
[6] WHO/OIE. “Echinococcosis in humans:
clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell MA, Meslin FX,
Pawlowski ZS, eds. Manual on echinococcosis in humans and animals: a public
health problem of global Concern.” Paris: World Organization for Animal
Health, pp. 20-66, 2001.
[7] WHO Informal Working Group.
International classification of ultrasound images in cystic echinococcosis for
application in clinical and field epidemiological settings. Acta Tropica 2003;
85: 253-261
[8] P Aeberhard, R Fuhrimann, P Strahm,
et al. “Surgical treatment of hydatid disease of the liver: an experience from
outside the endemic area.” Hepatogastroenterology, vol. 43, no. 9, pp.
627-636, 1996. View at: PubMed
[9] S Demirci, S Eraslan, E Anadol, et
al. “Comparison of the results of different surgical techniques in the
management of hydatid cyst of the liver.” World J Surg, vol. 13: 88-90.
View at: Publisher Site | PubMed
[10] P Magistrelli, R Masetti, R Coppola,
et al. “Surgical treatment of hydatid disease of the liver. A 20-year
experience.” Arch Surg, vol. 126, no. 4, pp. 518-522, 1991. View at: Publisher
Site | PubMed
[11] M Safioleas, E Misiakos, C Manti, et
al. “Diagnostic evaluation and surgical management of hydatid disease of the
liver.” World J Surg, vol. 18, no. 6, pp. 859-865, 1994. View at: Publisher Site | PubMed
[12] Queiroz-Medeiros M, Menezes-Silva A,
Raposo-D’Almeida, et al. “Surgical treatment of hepatic hydatid disease.”
European IHPBA Congress, Hamburg 97, Monduzzi editore, International
Proceedings Division, Bologna pp. 229-233, 1997.
[13] C Franchi, B Di Vico, A Teggi
“Long-term evaluation of patients with hydatidosis treated with benzimidazole
carbamates.” Clin Infect Dis, vol. 29, no. 2, pp. 304-309, 1999. View
at: Publisher Site | PubMed
[14] O F Senyüz, E Yeşildag, S Celayir “Albendazole therapy in the treatment
of hydatid liver disease.” Surg Today, vol. 31, no. 6, pp. 487-491, 2001. View at: Publisher Site | PubMed
[15] A G Saimot “Medical treatment of
liver hydatidosis.” World J Surg, vol. 25, no. 1, pp. 15-20, 2001. View
at: Publisher Site | PubMed
[16] H Wen, P F Zou, W G Yang, et al.
“Albendazole chemotherapy for human cystic and alveolar echinococcosis in
north-western China.” Trans R Soc Trop Med Hyg, vol. 88, no. 3, pp.
340-343, 1994. View at: Publisher
Site | PubMed
[17] D Anadol, U Ozçelik, N Kiper, et al.
“Treatment of hydatid disease.” Paediatr Drugs, vol. 3, no. 2, pp.
123-135, 2001. View at: Publisher Site | PubMed
[18] M S Khuroo, S A Zargar, R Mahajan “Echinococcus
granulosus cysts in the liver: Management with percutaneous drainage.” Radiology,
vol. 180, no. 1, pp. 141-145, 1991. View at: Publisher Site | PubMed
[19] G Simonetti, S Profili, G L
Sergiacomi, et al. “Percutaneous treatment of hepatic cysts by aspiration and
sclerotherapy.” Cardiovasc Intervent Radiol, vol. 16, no. 2, pp. 81-84,
1993. View at: Publisher
Site | PubMed
[20] M A Tolga Muftuoglu, N Koksal, U
Topaloglu “The role of omentoplasty in the surgical management of remnant
cavity in hepatic hydatid cyst.” HPB (Oxford), vol. 7, no. 3, pp.
231-234, 2005. View at: Publisher
Site | PubMed
[21] Christos Dervenis, Spiros Delis,
Costas Avgerinos, et al. “Changing concepts in the management of liver hydatid
disease.” J Gastrointest Surg, vol. 9, no. 6, pp. 869-877, 2005. View
at: Publisher
Site | PubMed
[22] B Gollackner, F Längle, H Auer, et
al. “Radical surgical therapy of abdominal cystic hydatid disease: Factors of
recurrence.” World J Surg, vol. 24, no. 6, pp. 717-721, 2000.
View at: Publisher
Site | PubMed
[23] A Cirenei, I Bertoldi “Evolutions of
surgery for liver hydatidosis from 1950 to today: Analysis of a personal
experience.” World J Surg, vol. 25, no. 1, pp. 87-92, 2001. View at: Publisher Site | PubMed
[24] Metin Ertem, Tayfun Karahasanoglu,
Nihat Yavuz, et al. “Laparoscopically treated liver hydatid cysts.” Arch
Surg, vol. 137, no. 10, pp. 1170-1173, 2002. View at: Publisher Site | PubMed
[25] P K Chowbey, S Shah, R Khullar, et
al. “Minimal access surgery for hydatid cyst disease: laparoscopic,
thoracoscopic, and retroperitoneoscopic approach.” J Laparoendosc Adv Surg
Tech, vol. 13, no. 3, pp. 159-165, 2003. View at: Publisher Site | PubMed
[26] B Gloor, Q Ly, D Candinas “Role of
laparoscopy in hepatic cyst surgery.” Dig Surg, vol. 19, no. 6, pp.
494-499, 2002. View at: Publisher
Site | PubMed
[27] D R Smego, R A Smego Jr “Hydatid
cyst: Pre-operative sterilization with mebendazole.” South Med J, vol.
79, no. 7, pp. 900-901, 1986. View at: PubMed
[28] Vasudevan Baskaran, Pradeep Kumar
Patnaik “Feasibility and safety of laparoscopic management of hydatid disease
of the liver.” JSLSL, vol. 8, no. 4, pp. 359-363, 2004. View at: PubMed
[29] C Manterola, O Fernández, S Muñoz, et
al. “Laparoscopic pericystectomy for liver hydatid cysts.” Surg Enosc,
vol. 16, no. 3, pp. 521-524, 2002. View at: Publisher Site | PubMed
[30] R Seven, E Berber, S Mercan, et al.
“Laparoscopic treatment of liver hydatid cyst.” Surgery, vol. 128, no.
1, pp. 36-40, 2000. View at: Publisher
Site | PubMed
[31] A Alper, A Emre, K Acarli, et al.
“Laparoscopic treatment of hepatic hydatid disease.” J Laparoendosc Surg,
vol. 6, no. 1, pp. 29-33, 1996. View at: Publisher Site | PubMed
[32] A Alper, A Emre, H Hazar, et al.
“Laparoscopic surgery of hepatic hydatid disease: Initial results and early
follow-up of 16 patients.” World J Surg, vol. 19, no. 5, pp. 725-728,
1995. View at: Publisher
Site | PubMed
[33] J L Dawson, J D Stamatakis, M D
Stringer, et al. “Surgical treatment of hepatic hydatid disease.” Br J Surg,
vol. 75, no. 10, pp. 946-950, 1988. View at: Publisher Site | PubMed
[34] T D Sielaff, B Taylor, B Langer
“Recurrence of hydatid disease.” World J Surg, vol. 25, no. 1, pp.
83-86, 2001. View at: Publisher
Site | PubMed
[35] S Alfieri, G B Doglietto, F Pacelli,
et al. “Radical surgery for liver hydatid disease: a study of 89 consecutive
patients.” Hepatogastroenterology, vol. 44, no. 14, pp. 496-500, 1997.
View at: PubMed
[36] M A Silva, D F Mirza, S R Bramhall,
et al. “Treatment of hydatid disease of the liver. Evaluation of a UK
experience.” Dig Surg, vol. 21, no. 3, pp. 227-233, 2004. View at: Publisher Site | PubMed
[37] E Moreno González, P Rico Selas, B
Martínez, et al. “Results of surgical treatment of hepatic hydatidosis: current
therapeutic modifications.” World J Surg, vol. 15, no. 2, pp. 254-263,
1991. View at: Publisher
Site | PubMed
[38] A Alper, O Ariogul, A Emre, et al.
“Choledochoduodenostomy for intrabiliary
rupture of hydatid cysts of the liver.” Br J Surg, vol. 74, no. 4, pp.
243-245, 1987. View at: Publisher
Site | PubMed
[39] Omer Topcu, Zeynep Sumer, Ersin Tuncer,
et al. “Efficacy of chlorhexidine gluconate during surgery for hydatid cyst.” World
J Surg, vol. 33, no. 6, pp. 1274-1280, 2009. View at: Publisher Site | PubMed
[40] N Ormeci, I Soykan, A Bektas, et al.
“A new percutaneous approach for the treatment of hydatid cysts of the liver.” Am
J Gastrenterol, vol. 96, no. 7, pp. 2225-2230, 2001. View at: Publisher Site | PubMed
[41] Akhan O, Ozmen MN. Percutaneous
treatment of liver hydatid cysts. Eur J Radiol 1999; 32: 76-85.
[42] B Ustünsöz, O Akhan, M A Kamiloğlu, et al. “Percutaneous treatment of
hydatid cysts of the liver: long-term results.” AJR Am J Roentgenol,
vol. 172, no. 1, pp. 91-96, 1999. View at: Publisher Site | PubMed
[43] Osman Yüksel, Nusret Akyürek, Tolga
Sahin, et al. “Efficacy of radical surgery in preventing early local recurrence
and cavity related complications in hydatic liver disease.” J Gastrointest
Surg, vol. 12, no. 3, pp. 483-489, 2008. View at: Publisher Site | PubMed
[44] M S Khuroo, N A Wani, G Javid, et al.
“Percutaneous drainage compared with surgery for hepatic hydatid cysts.” N
Engl J Med, vol. 337, no. 13, pp. 881-887, 1997. View at: Publisher Site | PubMed
[45] O Akhan, A Dincer, A Gököz, et al.
“Percutaneous treatment of abdominal hydatid cysts with hypertonic saline and
alcohol. An experimental study in sheep.” Invest Radiol, vol. 28, no. 2,
pp. 121-127, 1993. View at: Publisher Site | PubMed
[46] O Akhan, M N Ozmen, A Dinçer, et al.
“Liver hydatid disease: long-term results of percutaneous treatment.” Radiology,
vol. 198, no. 1, pp. 259-264, 1996. View at: Publisher Site | PubMed
[47] V Tasev, V Dimitrova, K Draganov, et
al. “Hepatic echinococcosis: radical or conservative surgical treatment.” Khirurgiia
(Sofiia), vol. 58, no. 2, pp. 10-13, 2002. View at: PubMed
[48] M Moumen, M E Elalaoui, M Mehhane, et
al. “Resection of the prominent dome of a hydatid cyst of the liver: about 360
cases.” J Chir (Paris), vol. 127, no. 2, pp. 83-86, 1990. View at: PubMed
[49] G Castellano, D Moreno-Sanchez, J
Gutierrez, et al. “Caustic sclerosing cholangitis. Report of four cases and a
cumulative review of the literature.” Hepatogastroenterology, vol. 41,
no. 5, pp. 458-470, 1994. View at: PubMed
[50] Milicevic M “Hydatid Disease. In
Surgery of the Liver and Biliary Tract.” edited by Blumgart LH; Edinburgh:
Churchill Livingstone, pp. 1121 1150, 1994.
[51] H Besim, K Karayalçin, O Hamamci, et
al. “Scolicidal agents in hydatid cyst surgery.” HPB Surg, vol. 10, no.
6, pp. 347-351, 1998. View at: Publisher
Site | PubMed
[52] C Kayaalp, M Balkan, C Aydin, et al.
“Hypertonic saline in hydatid disease.” World J Surg, vol. 25, no. 8,
pp. 975-979, 2001. View at: Publisher
Site | PubMed
[53] G Castellano, D Moreno-Sanchez, J
Gutierrez, et al. “Caustic sclerosing cholangitis. Report of four cases and a
cumulative review of the literature.” Hepatogastroenterology, vol. 41,
no. 5, pp. 458-470, 1994. View at: PubMed
[54] J W Schaefer, M Y Khan
“Echinococcosis (hydatid disease): Lessons from experience with 59 patients.” Rev
Infect Dis, vol. 13, no. 2, pp. 243-247, 1991. View at: Publisher Site | PubMed
[55] Saidi, F. (1976). Surgery of hydatid
disease. Saunders company London-Philadelphia-Toronto. View at: Publisher Site | PubMed
[56] Reeder MM, Palmer PES “The Radiology
of Tropical Diseases with Epidemiological, Pathological and Clinical
Correlation.” Baltimore, Williams and Wilkins, 1981.
[57] R W Ammann, J Eckert “Cestodes
Echinococcus.” Gastroenterol Clin North Am, vol. 25, no. 3, pp. 655-689,
1996. View at: Publisher
Site | PubMed
[58] C A Pissiotis, J V Wander, R E Condon
“Surgical treatment of hydatid disease.” Arch Surg, vol. 104, no. 4, pp.
454-459, 1972. View at: Publisher
Site | PubMed
[59] A A Balik, M Başoğlu, F Celebi, et al. “Surgical treatment of hydatid disease
of the liver.” Arch Surg,
vol. 134, no. 2, pp. 166-169, 1999. View at: Publisher Site | PubMed
[60] Vagioanos C, Androulakis JA
“Capsectomy and drainage in hepatic hydatidosis.” Dig Surg, vol. 14, pp.
241-244, 1997.
[61] R Dumas, P Le Gall, P Hastier, et al.
“The role of endoscopic retrograde cholangiopancreatography in the management
of hepatic hydatid disease.” Endoscopy, vol. 31, no. 3, pp. 242-247,
1999. View at: Publisher
Site | PubMed
[62] A N Rodriguez 1, A L Sánchez del Río,
L V Alguacil, et al. “Effectiveness of endoscopic sphincterotomy in
complicated hepatic hydatid
disease.” Gastrointest Endosc, vol. 48, no. 6, pp. 593-597, 1998.
View at: Publisher
Site | PubMed
[63] M L Vignote, G Miño, M de la Mata, et
al. “Endoscopic sphincterotomy in hepatic hydatid disease open to the biliary
tree.” Br J Surg, vol. 77, no. 1, pp. 30-31, 1990. View at: Publisher Site | PubMed
[64] Roland Chautems, Leo Buhler, Benjamin
Gold, et al. “Long term results after complete or incomplete surgical resection
of liver hydatid disease.” Swiss Med Wkly, vol. 133, no. 17-18, pp.
258-262, 2003. View at: Publisher
Site | PubMed
[65] Metin Kapan, Selin Kapan, Ertugrul
Goksoy, et al. “Postoperative recurrence in hepatic hydatid disease.” J
Gastrointest Surg, vol. 10, no. 5, pp. 734-739, 2006. View at: Publisher Site | PubMed
[66] J Prousalidis, C H Kosmidis, E
Fahantidis, et al. “Surgical treatment of multiple cystic echinococcosis.” HPB
(Oxford), vol. 6, no. 2, pp. 110-114, 2004. View at: Publisher Site | PubMed
[67] Yi-Biao He, Lei Bai, Yi Jiang, et al.
“Application of a three-dimensional reconstruction technique in liver
autotransplantation for end-stage hepatic alveolar echinococcosis.” J
Gastrointest Surg, vol. 19, no. 8, pp. 1457-1465, 2015. View at: Publisher Site | PubMed
[68] Solange Bresson-Hadni, Oleg
Blagosklonov, Jenny Knapp, et al. “Should possible recurrence of disease
contraindicate liver transplantation in patients with end-stage alveolar
echinococcosis? A 20-year follow-up study.” Liver Transpl, vol. 17, no.
7, pp. 855-865, 2011. View at: Publisher
Site | PubMed
[69] Hai Wang, Qiaoyu Liu, Zhaoming Wang, et al. “Clinical outcomes of Ex Vivo liver resection and liver autotransplantation for hepatic alveolar echinococcosis.” J Huazhong Univ Sci Technol Med Sci, vol. 32, no .4, pp. 598-600 View at: Publisher Site | PubMed
[70] W Patkowski, M Kotulski, P Remiszewski, et al. “Alveococcosis of the liver - strategy of surgical treatment with special focus on liver transplantation.” Transpl Infect Dis, vol. 18, no. 5, pp. 661-666, 2016. View at: Publisher Site | PubMed
