Received: Tue 16, Jul 2024
Accepted: Tue 30, Jul 2024
Abstract
Background: Lumbar hernias are rare abdominal wall hernias that may occur spontaneously or at the site of previous surgical procedures. Their infrequent nature can limit immediate recognition and standardization of repair. Furthermore, those sustained after lateral surgical approach for thoracolumbar spine surgery are sparsely documented.
Case Description: Here we report a case of a 61-year-old woman who presented with a symptomatic lumbar hernia nine years after undergoing polyether ether ketone (PEEK) cage fusion for L4/L5 spondylolisthesis through a lateral retroperitoneal approach. She presented with left flank pain and a vaguely palpable mass, and the diagnosis was confirmed by computed tomography (CT) imaging. The patient underwent uneventful open repair with intramuscular mesh placement and bilateral soft tissue flap advancement through a posterolateral oblique incision.
Conclusion: Symptoms of flank pain and swelling should raise concern for the diagnosis of lumbar hernia in patients who have undergone prior thoracolumbar surgery through a retroperitoneal approach. Cross-sectional imaging often confirms the diagnosis. Timely recognition reduces the risk of the progression of the defect and the risk of potential complications. Although a number of techniques have been described for repair of these defects, an open approach with intramuscular mesh placement offers excellent short-term results.
Keywords
Lumbar hernia, open lumbar hernia repair, lumbar hernia symptoms, lumbar hernia location, lumbar hernia anatomy
1. Introduction
Although initially described in 1672, lumbar hernias remain a rare surgical disorder. Factors contributing to their rare nature may include low incidence, vague or mild symptoms, and lack of detection on physical examination. Approximately 80% of lumbar hernias are acquired, and 25% are related to prior surgery or trauma [1]. Retroperitoneal exposure is frequently utilized for surgical procedures such as nephrectomy, aortic aneurysm repair, iliac bone graft harvesting, and latissimus dorsi myocutaneous flap harvesting for post-mastectomy reconstruction. Lumbar hernias following these procedures are less frequently documented [2].
Despite an increase in retroperitoneal procedures, the incidence of lumbar hernias has not increased, which may be due to underdiagnosis. Lumbar hernias may be misattributed as sciatica, lipoma, abscess, or abdominal wall atrophy secondary to surgical denervation [2]. Identification with clinical examination reveals a reducible flank mass with increasing prominence during valsalva maneuvers. Ultrasound and CT scans may confirm the diagnosis and determine the extent of herniation [3]. The surgical approach to lumbar hernia repair is also variable. Generally, repair may be conducted with an open or laparoscopic approach with or without mesh and with various means of mesh fixation. This may be performed posteriorly with the patient in a lateral position or through an anterior retroperitoneal approach. Surrounding bony structures promote obstacles to either. An open technique may be preferred for cases of recurrent lumbar herniation [3, 4].
Herein, we report a case for an incisional lumbar hernia repair implementing an open lateral approach to the thoracolumbar spine. We report the following case in accordance with the CARE reporting checklist.
2. Case Presentation
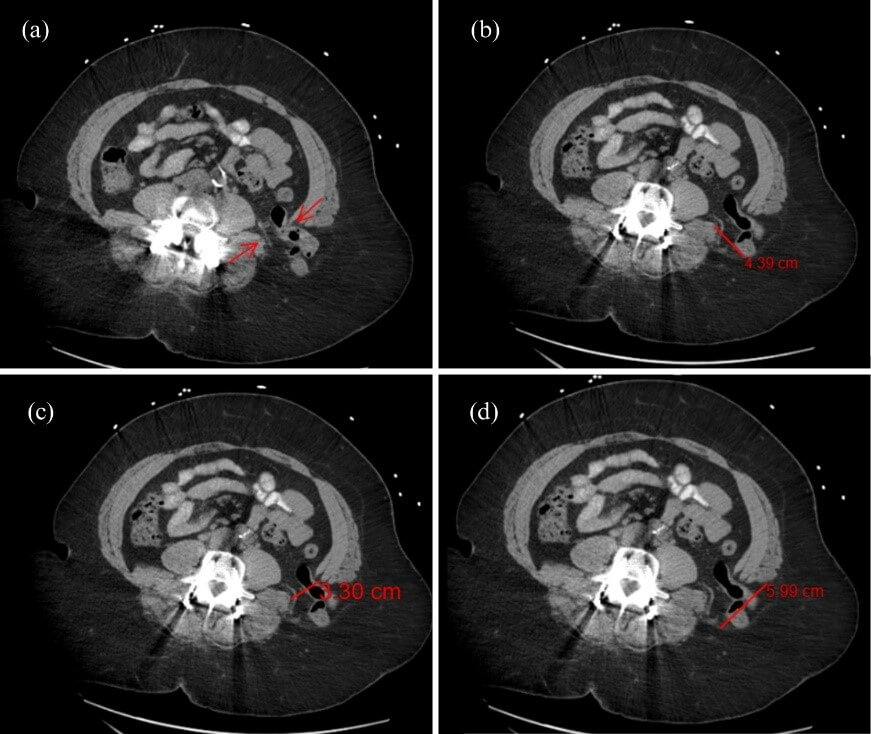
A 61-year-old woman with a known medical history of coronary artery disease, asthma, and a BMI of 41.33 (kg/m2) presented to the emergency department with acute left-sided chest and upper quadrant abdominal pain with radiation to her left lateral back. Two hours before the onset of symptoms, she reported heavy lifting at work. Her surgical history was significant for childhood umbilical hernia repair, tonsillectomy, incision and drainage of abdominal wall abscess, and repair of spondylolisthesis and spinal stenosis via interbody fusion. Nine years before presentation, she underwent minimally invasive exposure of the lumbar spine via a left lateral retroperitoneal approach with polyether ether ketone (PEEK) interbody fusion cage of L4-L5 and bilateral pedicle screw fixation. On examination, the patient was hemodynamically stable with intermittent tachypnea. The abdomen was obese and soft, with mild tenderness to palpation and a vaguely palpable firm mass within the left flank. CT scan of the abdomen and pelvis with oral contrast showed a 3.3 cm defect within the posterolateral left abdominal wall above the iliac wing (Figure 1c). The size of the hernia orifice measured 5.99 cm × 4.39 cm at maximum axial transverse and anteroposterior diameters via preoperative scan (Figures 1b & 1d). Contents of the hernia included intraperitoneal fat and a loop of descending colon without signs of incarceration, strangulation, or obstruction (Figure 2). The patient was stable and was referred to the general surgery clinic for evaluation for repair. After discussing the risks, benefits, and appropriate preoperative evaluation, she was seen and scheduled for elective repair. Surgery was scheduled for an open lumbar hernia repair with intramuscular mesh placement.
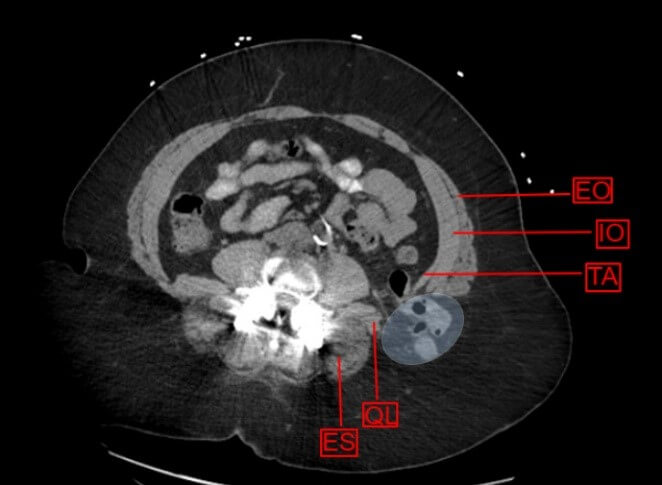
EO: External Oblique; IO: Internal Oblique; TA: Transversus Abdominus; QL: Quadratus Lumborum; ES: Erector Spinae.
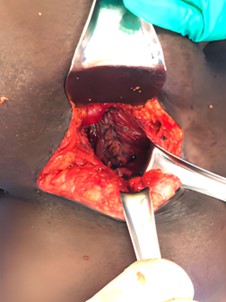
The patient underwent general endotracheal anesthesia, which was maintained over three hours with sevoflurane inhaled anesthetic. Total procedural duration, as counted from the time of the first incision, was approximately two hours. The patient was repositioned in the right lateral decubitus position using a bean bag for support with an overhead arm board. The bed was draped to allow for right-sided flexion to improve exposure of the lumbar region. The left flank was prepped and draped in standard surgical fashion. An oblique incision was created in the left flank between the 12th rib and iliac crest using a bovie electrocautery at the site of the previous scar with continued dissection through the skin and subcutaneous tissue. The soft tissue overlying the hernia sac was excised, and the hernia sac was identified and dissected down to the level of the myofascial orifice. The hernia sac was excised, and further dissection was performed deep to the latissimus and external oblique muscles, where the medial internal oblique muscle and corresponding fascia were freed from overlying tissue with bovie cautery. The lateral quadratus lumborum and paraspinous muscles were also dissected away from overlying muscular tissue, thus creating a space for intramuscular mesh placement. The internal oblique fascia and muscle were secured to the quadratus lumborum muscle using interrupted #0 nonabsorbable monofilament (Novafil ™, Ethicon ©, Somerville, NJ) sutures (Figure 3).
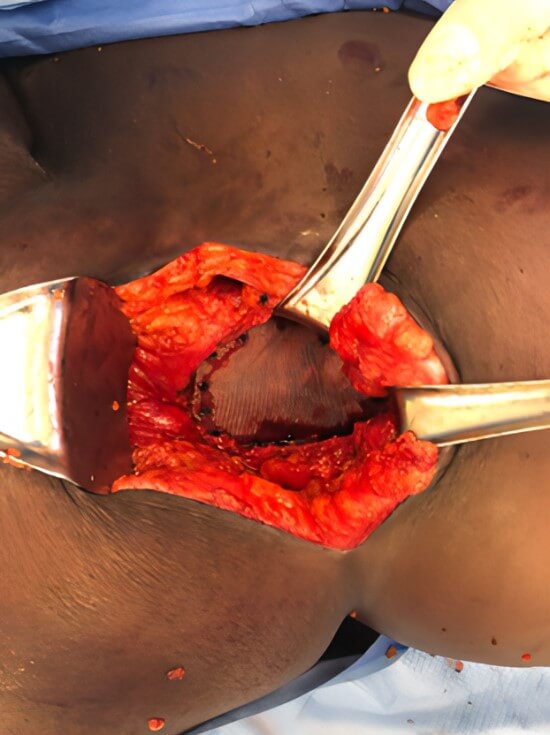
Polypropylene monofilament mesh measuring 26 cm × 36 cm was cut to shape making sure its borders exceeded the hernia orifice by at least 3 cm in all directions (Bard® Mesh, Becton, Dickinson and Company ©, Franklin Lakes, NJ). The appropriately sized mesh was placed between the muscle layers and secured using absorbable tacks (Figure 4). One gram of topical cefazolin powder was applied to the visible mesh surface as routine practice. The latissimus was then secured to the aponeurosis of the external oblique using interrupted 1-0 nonabsorbable monofilament (Novafil ™ , Ethicon ©, Somerville, NJ) suture. A 15 Fr closed negative pressure drain (Blake ™ Silicone Drain, Ethicon ©, Somerville, NJ) was placed in the subcutaneous space, brought out of the skin through a stab incision, and connected to a bulb suction reservoir. The drain was secured to the skin at its exit site using a 3-0 nylon (Ethilon ™, Ethicon ©, Somerville, NJ) suture. The skin was then reapproximated using 3-0 absorbable, synthetic sutures (Vicryl ™, Ethicon ©, Somerville, NJ) and staples. A dry sterile dressing was applied. The patient tolerated the procedure well, and there were no complications.
Post-operative recovery was uneventful. She was discharged home on postoperative day two with no documented complications. At discharge, less than 200 cc of drain output was documented since its placement. Classical hernia follow-up was instituted thereafter. Postoperative follow-up was completed through the outpatient service at 2 weeks, 6 months, 3 months, and 1 year thereafter. Regular physical examination was routinely performed to exclude post-operative complications. On postoperative day 13, she was seen in the office for staple and drain removal as the output was negligible (<30 cc/day). She continued with interval outpatient follow-up. As of the time of publication, the patient is more than one-year status post-repair without clinical evidence of recurrence or complication.
3. Discussion
3.1. Anatomy
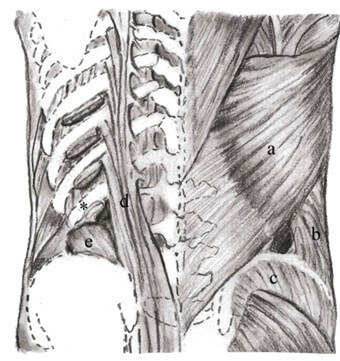
Lumbar hernias are uncommon clinical entities diagnosed infrequently, accounting for <2% of all abdominal wall hernias [5]. Lumbar hernias are posterolateral abdominal wall defects that occur anywhere between the 12th rib and iliac crest. Two well-defined areas of weakness exist in this region: the inferior lumbar triangle described by Petit in 1783 and the superior lumbar hernia triangle by Petit in 1866. The superior lumbar hernia, also called Grynfeltt hernia, refers to a hernia that protrudes through the superior lumbar triangle in the lumbar region. The superior triangle is an inverted triangle superiorly bound by the lower border of the 12th rib and the inferior posterior serratus muscle, medially bound by the erector spinae muscle and laterally bound by the internal oblique muscle. The transversalis fascia comprises the floor, while the external oblique and latissimus set the roof of the triangle. The inferior or Petit triangle is an upright triangular space that is bound by the iliac crest inferiorly, the lateral-anterior edge by the posterior border of the external abdominal oblique muscle, and medial-posteriorly by the lateral border of the latissimus dorsi muscle posteriorly (Figure 5) [5, 6].
3.2. Etiology
Ninety-five percent of lumbar hernias occur in these two anatomic locations of the lumbar wall [4]. Herniation occurs most frequently in the superior triangle due to its greater surface area when compared to the inferior triangle, making Grynfelt-Lesshaft hernias more common than Petit. Secondary lumbar hernias account for 25% of acquired lumbar hernias and are either iatrogenic (often at surgical incision sites), traumatic, or follow recurrent infection or inflammation [7]. Kelton first described incisional acquired lumbar hernias in 1939 [8]. Iatrogenic causes include injury to the retroperitoneal fascia via manipulation during urologic/kidney surgery, aortic repair, radical cystectomy, and iliac bone graft harvesting [5, 8]. These procedures, in addition to latissimus dorsal flaps for breast reconstruction, are reported to be at the most risk of developing Grynfeltt hernias [9]. Prevalence of herniation after lumbotomy involves muscular atrophy due to subcostal nerve dissection, resulting in gradual thinning of the muscle and fascia predisposing to hernia appearance [10]. Traumatic lumbar hernias are less documented, with 164 cases reported in the literature between 1990 and 2021 [11].
3.3. Clinical Presentation & Recognition
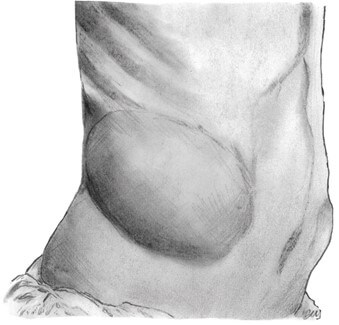
The typical presentation can be straightforward with what can be informally described as a "bulge" in the lumbar region that displays both a visible and palpable impulse with valsalva (Figure 6). The bulge may disappear in the supine or lateral position and become prominently observed on standing and coughing [12]. CT scan is the gold standard of diagnosis, conferring 98% sensitivity based on the ability to distinguish between fascial and muscular layers and to deduce the nature of the herniated content [13]. Delayed identification leads to the progression of the wall defect and, therefore, more significant overall herniation once identified. Due to atypical clinical symptoms, prolonged diagnosis may lead to eventual obstruction or strangulation, albeit rare. The risk for incarceration is less than 10% due to the atypically wide neck of the defect and anatomic location [3, 14].
Risk factors for developing a primary lumbar hernia are like other ventral hernias: obesity, connective tissue disease, poor nutritional status, and conditions that increase intra-abdominal pressure, such as persistent coughing. Furthermore, deterministic factors for superior lumbar herniations revolve around i) the size of the triangle, ii) the length and angulation of the 12th rib, and iii) the size of quadratus lumborum and serratus posterior inferior muscles. Bearing this in mind, hernias are primarily encountered in short, obese individuals with horizontal slanting ribs and triangles with large surface areas [15]. Adding to an individual's habitus and build would be previous retroperitoneal fascial and muscular manipulations or other trauma like high-energy torso injuries (i.e., motor vehicle accidents) [11, 16].
3.4. Treatment
Surgical repair is the only method for the treatment of lumbar hernias. However, the lack of collective experience of any single surgeon prevents procedural standardization and lack of consensus in the literature. The implicit technical difficulties, regardless of approach, faced in repairing a posterior abdominal wall defect can be attributed to location, lack of adequate fascia, weakness of surrounding tissue, and bony boundaries in proximity, limiting visualization of the defect [5, 6]. Small hernias (< 5 cm) with well-defined borders, normal lumbar anatomy, and without viscera content are good candidates for the preperitoneal and laparoscopic approaches. Laparoscopic repair has been primarily applied to small primary lumbar hernias due to the restriction in separating retroperitoneal space limited by the 12th rib and iliac crest [13, 14]. The first laparoscopic repairs were those by Heniford et al., Bickel et al., and Arca et al. in the late 1990s [17]. Primary repair with muscular layer approximation closes the defect but may result in high tension, severe pain, and a high recurrence rate (Dongguan). The first consistent series (20 patients) of direct primary lumbar hernia repairs was published by Light in 1983 [10, 17].
Based on a literature review, there is no ideal technique for repairing these types of hernias. Preoperative decision-making regarding repair should consider i) open vs laparoscopic approach, ii) the use of mesh, type, and location, and iii) if with use of mesh, the method of fixation. Previous studies have described many open approaches to lateral hernia repairs-including open midline or lateral approaches. Montelione et al. reported an algorithm for open retro-muscular lateral abdominal wall hernia repairs applied to 464 cases over eight years [18]. Determination of patient positioning and surgical access incisions were based on midline involvement and defect distance from the midline. In brief, isolated lateral hernias located >12 cm from the linea alba (like those of the lumbar region) may suffer from inadequate exposure through midline incisions. Based on the described algorithm, lateral decubitus positioning with a lateral incision allows for substantial ease of exposure and access to complex lateral aspects of repair [16]. This algorithm seems comparable to the Moreno Egea therapeutic classification system, where both require only two deterministic variables [10, 18]. Their findings coincide nicely with the authors’ reported approach and 1-year postoperative outcomes.
For complex incisional hernias, the Rives-stoppa retro-muscular repair (RSRT) seems to be an excellent option for the open technique of prosthetic repair, with recurrence rates of 0% to 18% [19]. The first series regarding the use of synthetic mesh was made by Hafner et al. in 1963 [10]. Regarding Grynfeltt and Petit’s hernias, the mesh (usually polypropylene or dacron) should be placed according to tension-free principles and the Rives-Stoppa technique. In adults, the Rives-Stoppa retro-muscular technique (RSRT) is the technique of choice when repairing > 5 cm midline hernias [18-20]. The authors’ personal experience supports great results with modified Rives-Stoppa repair for incisional hernias. Through this approach, critical elements to successful herniorrhaphy are accomplished: i) provision of mechanical strength via prosthesis to counteract intrabdominal fluctuations and ii) large mesh with extensive overlap of fascial edges permits for a tension-free closure [19]. Albeit a lumbar herniorrhaphy, the RSRT was mirrored in the following fashion: The securing of the internal oblique to the quadratus lumborum forms the innermost muscular layer, and the external oblique secured to the latissimus forms the outermost muscular layer between which polypropylene mesh was placed. This technique favors layered reconstruction, which restores the anatomical and physiological properties of the abdominal wall. Laparoscopic repair was considered, but an open approach was chosen given the concern for intraperitoneal injury and probable intraoperative difficulty.
For the matter of fixation-it is also debated whether fixation is at all necessary. There is a risk for recurrence, given the lack of fascial integrity surrounding the lumbar defect supporting the use of mesh [7, 19, 20]. In this case, to prevent mesh migration in the early postoperative period, absorbable tacks were used for relatively quick incorporation with equal consideration given to fixation sutures-both incur similar risks for inadvertent injury.
4. Conclusion
Our open technique avoids a transabdominal approach and thereby avoids the risk of injury to the small bowel or other structures. Further, it allows for mesh reinforcement of the defect without requiring intraperitoneal mesh placement, removal of redundant skin, optimization of cosmesis, and minimization of the risk of post-operative seroma formation.
In conclusion, open repair with intramuscular mesh placement is an effective treatment for durable repair with minimal risk of complications. This technique offers many potential benefits compared to minimally invasive or transabdominal approaches.
Acknowledgments
We gratefully thank all who contributed to this patient's care and recovery. Niagara Falls Memorial Medical Center and the Department of Surgery supported this work.
Author Contributions
Conception and design: P. Muscarella II, MD. Administrative Support: S. Jensen, MD. Provision of study materials or patients: None. Collection & Assembly of Data: None. Data analysis and interpretation: None. Manuscript writing: all authors. Final approval of manuscript: all Authors.
Reporting Checklist
The authors have completed the CARE reporting checklist.
Peer Review File
Available for review at the time of publication.
Conflicts of Interest
None.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). According to the Niagara Falls Memorial Medical Center ethics committee/institutional review board, publication of this case report and accompanying images was waived from patient consent.
REFERENCES
[1] Lorenzo
Capasso, Gaetano Romano, Antonio Alderisio, et al. “Primary acquired Grynfeltt
Hernia: two new cases and literature review.” Ann Ital Chir, vol. 89,
pp. 255-260, 2018. View at: PubMed
[2] Marc
Rafols, Daniel Bergholz, Anthony Andreoni, et al. “Bilateral Lumbar Hernias
Following Spine Surgery: A Case Report and Laparoscopic Transabdominal Repair.”
Case Rep Surg, vol. 2020, pp. 8859106, 2020. View at: Publisher
Site | PubMed
[3] Atul
K Madan, Craig A Ternovits, Karen E Speck, et al. “Laparoscopic Lumbar Hernia
Repair.” Am Surg, vol. 72, no. 4, pp. 318-321, 2006. View at: PubMed
[4] Dimitrios
Stamatiou, John E Skandalakis, Lee J Skandalakis, et al. “Lumbar Hernia:
Surgical Anatomy, Embryology, and Technique of Repair.” Am Surg, vol.
75, no. 3, pp. 202-207, 2009. View at: PubMed
[5] Ronggui
Lin, Tianhong Teng, Xianchao Lin, et al. “Sublay repair for primary superior
lumbar hernia with the Kugel patch.” ANZ J Surg, vol. 90, no. 5, pp.
776-780, 2020. View at: Publisher
Site | PubMed
[6] Giuseppe
Cavallaro, Arash Sadighi, Claudia Paparelli, et al. “Anatomical and Surgical
Considerations on Lumbar Hernias.” Am Surg, vol. 75, no. 12, pp.
1238-1241, 2009. View at: PubMed
[7] Rafael
García-Cañas “An Extremely Rare Case Report of a Retroperitoneal Hernia
Following Spinal Surgery.” Journal of Surgical Case Reports and Images,
vol. 5, no. 2, pp. 1-5, 2021. View at: Publisher Site
[8] Anthony
Pastore, Jonathan E Sobel “Lumbar hernia after iliac crest bone harvest.” JAAPA,
vol. 32, no. 1, pp. 33-34, 2019. View at: Publisher Site | PubMed
[9] Guido
Zanghi, Umberto Falzone, Enrico Sapienza, et al. “Grynfeltt-Lesshaft hernia
Personal experience of nine cases and a review of the literature.” Ann Ital
Chir, vol. 93, pp. 698-701, 2022. View at: PubMed
[10]
Alfredo Moreno-Egea, Enrique G Baena,
Miquel C Calle, et al. “Controversies in the Current Management of Lumbar
Hernias.” Arch Surg, vol. 142, no. 1, pp. 82-88, 2007. View at: Publisher Site | PubMed
[11]
Ioannis Tsouknidas, Nikolaos Tasis,
Maria Ioanna Antonopoulou, et al. “Traumatic lumbar hernia: A systematic review
of the literature.” Chin J Traumatol, vol. 27, no. 1, pp. 53-57, 2023.
View at: Publisher Site | PubMed
[12]
Ketan Vagholkar, Suvarna Vagholkar
“Open Approach to Primary Lumbar Hernia Repair: A Lucid Option.” Case Rep
Surg, vol. 2017, pp. 5839491, 2017. View at: Publisher Site | PubMed
[13]
Sebastian Suarez, Juan D Hernandez
“Laparoscopic repair of a lumbar hernia: report of a case and extensive review
of the literature.” Surg Endosc, vol. 27, no. 9, pp. 3421-3429, 2013.
View at: Publisher Site
| PubMed
[14]
Sharma P. Lumbar Hernia. Med J Armed
Forces India. 2009;65(2):178. View at: Publisher Site | PubMed
[15]
V Macchi, A Porzionato, A Morra, et
al. “The triangles of Grynfeltt and Petit and the lumbar tunnel: an
anatomo-radiologic study.” Hernia, vol. 21, no. 3, pp. 369-376, 2017.
View at: Publisher Site | PubMed
[16]
Nurkan Torer, Sedat Yildirim, Akin
Tarim, et al. “Traumatic lumbar hernia: Report of a case.” Int J Surg,
vol. 6, no. 6, pp. e57-e59, 2008. View at: Publisher Site | PubMed
[17]
A Moreno-Egea, J A Torralba-Martinez,
G Morales, et al. “Open vs laparoscopic repair of secondary lumbar hernias: A
prospective nonrandomized study.” Surg Endosc, vol. 19, no. 2, pp.
184-187, 2005. View at: Publisher Site | PubMed
[18]
Katherine C Montelione, Clayton C
Petro, David M Krpata, et al. “Open Retromuscular Lateral Abdominal Wall Hernia
Repair: Algorithmic Approach and Long-Term Outcomes at a Single Center.” J
Am Coll Surg, vol. 236, no. 1, pp. 220-234, 2023. View at: Publisher Site | PubMed
[19] Daniel Maman 1, Daniel Greenwald, Jonah Kreniske, et al. “Modified rives-stoppa technique for repair of complex incisional hernias in 59 patients.” Ann Plast Surg, vol. 68, no. 2, pp. 190-193, 2012. View at: Publisher Site | PubMed
[20] Peter Nau, Clancy J. Clark, Mason Fisher, et al. “Modified rives-stoppa repair for abdominal incisional hernias.” Health N Hav, vol. 2, no. 2, pp. 162-169, 2010. View at: Publisher Site
